In An Ionic Bond Electrons Are
listenit
Mar 14, 2025 · 6 min read
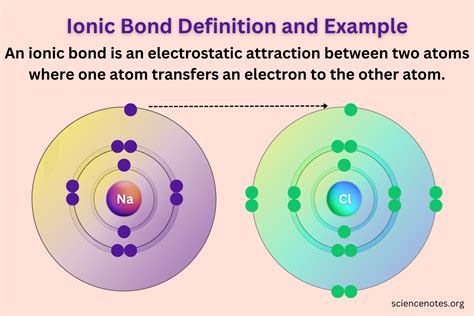
Table of Contents
In an Ionic Bond, Electrons Are…Transferred! Understanding the Fundamentals of Ionic Bonding
Ionic bonds, a cornerstone of chemistry, represent a fundamental type of chemical bonding characterized by the transfer of electrons from one atom to another. This transfer results in the formation of ions – atoms with a net electrical charge – and the subsequent electrostatic attraction between these oppositely charged ions creates the ionic bond. Understanding this fundamental process is crucial to grasping numerous chemical concepts and properties of ionic compounds.
The Electron Transfer Process: A Closer Look
The driving force behind ionic bond formation is the electrostatic attraction between positively and negatively charged ions. This attraction is powerful enough to overcome the repulsive forces between the positively charged nuclei of the involved atoms. But how does this transfer of electrons actually occur?
The key lies in the electronegativity of the atoms involved. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Atoms with significantly different electronegativities are more likely to form ionic bonds. Typically, this involves a metal with low electronegativity and a non-metal with high electronegativity.
Let's consider the classic example of sodium chloride (NaCl), or common table salt. Sodium (Na), an alkali metal, has a single electron in its outermost shell (valence shell). Chlorine (Cl), a halogen, has seven electrons in its valence shell. Chlorine has a much higher electronegativity than sodium.
During the formation of an ionic bond between sodium and chlorine:
- Sodium readily loses its single valence electron to achieve a stable, filled outer electron shell (octet rule). This process leaves sodium with one less electron than protons, resulting in a positive ion called a cation, denoted as Na⁺.
- Chlorine readily gains this electron, completing its own outer shell and achieving a stable octet. This addition of an electron gives chlorine one more electron than protons, resulting in a negative ion called an anion, denoted as Cl⁻.
The electrostatic attraction between the positively charged Na⁺ ion and the negatively charged Cl⁻ ion forms the ionic bond, holding the ions together in a crystalline structure. This structure is characterized by a regular arrangement of alternating cations and anions, maximizing the attractive forces and minimizing repulsive forces.
Beyond Sodium Chloride: Exploring Other Ionic Compounds
While NaCl serves as a perfect introductory example, ionic bonding occurs in a wide array of compounds involving various metals and nonmetals. The principles remain the same: a transfer of electrons from a less electronegative atom to a more electronegative atom, resulting in the formation of oppositely charged ions and a subsequent electrostatic attraction.
Consider magnesium oxide (MgO):
- Magnesium (Mg) loses two valence electrons to form Mg²⁺ (a cation with a 2+ charge).
- Oxygen (O) gains these two electrons to form O²⁻ (an anion with a 2- charge).
The strong electrostatic attraction between Mg²⁺ and O²⁻ forms the ionic bond in MgO.
Similarly, in aluminum chloride (AlCl₃):
- Aluminum (Al) loses three valence electrons to form Al³⁺.
- Three chlorine atoms each gain one electron to form three Cl⁻ ions.
The resulting electrostatic attraction between Al³⁺ and three Cl⁻ ions constitutes the ionic bonding in AlCl₃.
The number of electrons transferred depends on the number of valence electrons in the participating atoms and their desire to achieve a stable electron configuration (usually a full outer shell). This determines the charge of the resulting ions and the stoichiometry (ratio of atoms) in the ionic compound.
Properties of Ionic Compounds: A Direct Result of Ionic Bonding
The unique properties of ionic compounds are directly attributable to the nature of ionic bonding:
- High melting and boiling points: The strong electrostatic forces between the ions require significant energy to overcome, leading to high melting and boiling points.
- Crystalline structure: The regular arrangement of ions in a lattice structure contributes to the crystalline nature of ionic compounds.
- Brittleness: Applying stress can cause like charges to align, leading to repulsion and fracture.
- Solubility in polar solvents: Ionic compounds often dissolve readily in polar solvents like water, due to the interaction between the ions and the polar solvent molecules.
- Electrical conductivity: Ionic compounds conduct electricity when molten or dissolved in water, as the ions are free to move and carry charge. In their solid state, the ions are fixed in the lattice, preventing electrical conductivity.
Distinguishing Ionic Bonds from Covalent Bonds: Key Differences
It's crucial to differentiate ionic bonds from covalent bonds, another major type of chemical bond. While ionic bonds involve the transfer of electrons, covalent bonds involve the sharing of electrons between atoms.
| Feature | Ionic Bond | Covalent Bond |
|---|---|---|
| Electron Transfer/Sharing | Transfer of electrons | Sharing of electrons |
| Electronegativity Difference | Large | Small |
| Bonding Atoms | Typically metal and non-metal | Typically non-metals |
| Melting/Boiling Points | High | Relatively lower |
| Solubility | Often soluble in polar solvents | Varies, depends on polarity |
| Electrical Conductivity | Conducts when molten or dissolved | Generally does not conduct electricity |
Beyond the Basics: Exploring Complexities in Ionic Bonding
While the simple electron transfer model provides a good starting point, the reality of ionic bonding is often more nuanced. Factors such as polarization and lattice energy influence the properties of ionic compounds.
- Polarization: This refers to the distortion of the electron cloud of an anion by the nearby cation. Larger cations with lower charge density have less polarizing power than smaller, highly charged cations. Polarization can affect the properties of the ionic compound, sometimes leading to partial covalent character.
- Lattice Energy: This is the energy released when ions come together to form a crystalline lattice. It reflects the strength of the ionic bond and is influenced by factors like the charges and sizes of the ions. Higher lattice energy indicates stronger bonds and higher melting points.
Furthermore, some compounds exhibit characteristics of both ionic and covalent bonding, representing a spectrum rather than a strict dichotomy. These compounds are often described as having polar covalent character with some ionic contribution.
Applications of Ionic Compounds: A Wide Range of Uses
Ionic compounds have a vast array of applications across various industries and fields:
- Medicine: Many salts and ionic compounds are essential in medicine, serving as electrolytes, drugs, and components of various medical treatments.
- Industry: Ionic compounds find widespread use in manufacturing processes, such as the production of plastics, fertilizers, and detergents.
- Agriculture: Many fertilizers contain ionic compounds that provide essential nutrients for plant growth.
- Food Industry: Salt (NaCl) is a vital ingredient in food preservation and flavor enhancement.
- Everyday Life: Numerous common materials, such as table salt, baking soda, and plaster, are ionic compounds.
Conclusion: A Fundamental Concept with Broad Implications
In conclusion, ionic bonds are formed by the transfer of electrons from one atom to another, resulting in the formation of oppositely charged ions and the subsequent electrostatic attraction between them. This fundamental process underlies the properties and applications of a vast number of ionic compounds, shaping our world in countless ways. Understanding the intricacies of ionic bonding is crucial not only for mastering basic chemistry but also for advancing our knowledge in diverse scientific and technological fields. The continuous exploration of ionic bonding, its complexities, and its applications promises exciting discoveries and advancements in the future.
Latest Posts
Latest Posts
-
Vertex Angle Of An Isosceles Triangle
Mar 14, 2025
-
Which Subshell Is Represented By The Actinides Series
Mar 14, 2025
-
What Reagents Are Necessary To Perform The Following Reaction
Mar 14, 2025
-
What Element Has The Greatest Electronegativity
Mar 14, 2025
-
What Is The Overall Charge Of The Nucleus
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about In An Ionic Bond Electrons Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
