What Element Has The Greatest Electronegativity
listenit
Mar 14, 2025 · 6 min read
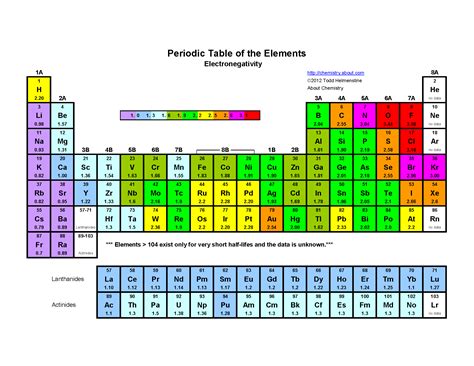
Table of Contents
What Element Has the Greatest Electronegativity?
Electronegativity, a fundamental concept in chemistry, describes an atom's ability to attract shared electrons in a chemical bond. Understanding electronegativity is crucial for predicting the polarity of bonds, the shapes of molecules, and the reactivity of chemical species. But which element reigns supreme in this atomic tug-of-war? The answer, unequivocally, is fluorine. This article delves deep into the reasons behind fluorine's exceptional electronegativity, exploring the underlying factors and comparing it to other highly electronegative elements. We’ll also explore the implications of high electronegativity and its impact on chemical behavior.
Understanding Electronegativity: A Deeper Dive
Before we crown fluorine the electronegativity champion, let's establish a firm understanding of the concept itself. Electronegativity isn't a directly measurable property like mass or charge. Instead, it's a relative measure, typically represented by numerical values on various scales, most notably the Pauling scale. These scales reflect the tendency of an atom to attract electrons towards itself when it is bonded to another atom.
Several factors contribute to an atom's electronegativity:
1. Nuclear Charge: The Stronger Pull
The positive charge of the nucleus plays a dominant role. A higher nuclear charge means a stronger electrostatic attraction for electrons. As we move across a period in the periodic table, the nuclear charge increases, leading to a general increase in electronegativity.
2. Atomic Radius: Distance Matters
The distance between the nucleus and the valence electrons is equally important. A smaller atomic radius means the valence electrons are closer to the positively charged nucleus, experiencing a stronger attractive force. As we move down a group in the periodic table, the atomic radius increases, leading to a decrease in electronegativity.
3. Shielding Effect: Inner Electrons' Influence
The shielding effect, caused by inner electrons repelling outer electrons, also plays a significant role. Inner electrons partially shield the outer electrons from the full attractive force of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the valence electrons, thereby decreasing electronegativity.
Fluorine: The Electronegativity King
Now, let's focus on fluorine. Its exceptional electronegativity is a consequence of the interplay of these three factors:
- High Nuclear Charge: Fluorine possesses a relatively high nuclear charge for its period.
- Small Atomic Radius: Fluorine has an extremely small atomic radius compared to other elements. Its valence electrons are exceptionally close to the nucleus, experiencing a potent electrostatic pull.
- Minimal Shielding: With only two inner electrons, the shielding effect in fluorine is minimal. The valence electrons are not significantly shielded from the nucleus's attractive force.
The combination of these factors results in fluorine having the highest electronegativity of all elements. Its powerful attraction for electrons dominates its interactions with other atoms.
Comparing Fluorine to Other Highly Electronegative Elements
While fluorine holds the top spot, several other elements exhibit high electronegativity:
-
Oxygen: Oxygen is the second most electronegative element. While its nuclear charge is lower than fluorine's, its smaller size compared to other elements in its period, and relatively weak shielding effect contribute to its high electronegativity. However, oxygen’s electronegativity is considerably lower than fluorine’s due to its larger atomic radius and stronger shielding effect compared to fluorine.
-
Chlorine: Chlorine, located below fluorine in the same group (halogens), exhibits high electronegativity. Its high nuclear charge contributes to this, but the increase in atomic radius and stronger shielding effect compared to fluorine significantly lowers its electronegativity.
-
Nitrogen: Nitrogen, a non-metal in group 15, also displays significant electronegativity. Its smaller size and relatively high nuclear charge contributes to its high electronegativity. However, compared to fluorine, oxygen, and chlorine, nitrogen possesses a significantly lower electronegativity due to a relatively larger atomic radius and increased shielding effect.
The gradual decrease in electronegativity as we move down Group 17 (halogens) — from fluorine to chlorine, bromine, and iodine — exemplifies the influence of increasing atomic radius and shielding effects on electronegativity.
Electronegativity and Chemical Bonding: A Powerful Influence
The high electronegativity of fluorine significantly impacts its chemical behavior and the nature of bonds it forms:
1. Polar Bonds: Unequal Sharing
Fluorine readily forms polar covalent bonds with other elements. Due to its strong pull on shared electrons, the electrons spend significantly more time closer to the fluorine atom. This creates a partial negative charge (δ-) on the fluorine atom and a partial positive charge (δ+) on the atom it is bonded to. This polarity is responsible for many of the unique properties of fluorine-containing compounds.
2. Ionic Bonds: Extreme Cases
When fluorine reacts with elements with significantly lower electronegativity (e.g., alkali metals), the difference in electronegativity is so substantial that electrons are essentially transferred completely from the less electronegative element to fluorine. This results in the formation of ionic bonds, producing ionic compounds like sodium fluoride (NaF).
3. Hydrogen Bonding: Special Interactions
Fluorine's high electronegativity also contributes to the formation of strong hydrogen bonds. When a hydrogen atom is bonded to a highly electronegative atom like fluorine, the hydrogen atom carries a significant partial positive charge. This positive charge attracts the lone pair of electrons on another highly electronegative atom, resulting in a strong dipole-dipole interaction known as a hydrogen bond. This is especially crucial in the properties of water and many biological molecules.
Implications of High Electronegativity
Fluorine's extreme electronegativity has widespread implications across various fields:
-
Inorganic Chemistry: Fluorine's high reactivity and strong bond formation make it crucial in the synthesis of various inorganic compounds, some with unique properties and applications.
-
Organic Chemistry: Organofluorine compounds, those containing carbon-fluorine bonds, are of considerable interest due to the stability, lipophilicity, and reactivity they often exhibit. They are commonly found in pharmaceuticals and materials science.
-
Material Science: Fluorine's ability to form strong bonds is exploited in various materials. For example, Teflon, a polymer of tetrafluoroethylene, demonstrates exceptional chemical inertness and non-stick properties due to the strong C-F bonds.
-
Nuclear Chemistry: Certain fluorine compounds are used in nuclear applications due to fluorine's ability to form stable bonds with various elements.
Conclusion: Fluorine's Reign Supreme
In summary, fluorine holds the title of the element with the greatest electronegativity. Its high nuclear charge, incredibly small atomic radius, and minimal shielding effect synergistically contribute to its unparalleled ability to attract electrons. This high electronegativity profoundly influences its chemical behavior, leading to the formation of polar bonds, ionic bonds, and strong hydrogen bonds. The implications of fluorine's exceptional electronegativity extend across diverse fields, highlighting its importance in chemistry, materials science, and other scientific disciplines. While other elements exhibit high electronegativity, none can match fluorine’s dominance in this atomic characteristic.
Latest Posts
Latest Posts
-
What Percent Of 40 Is 3
Mar 14, 2025
-
Square Root Of X 6 X
Mar 14, 2025
-
What Percent Of 80 Is 10
Mar 14, 2025
-
5 1 2 As An Improper Fraction
Mar 14, 2025
-
What Are The 3 Parts Of An Atp Molecule
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Element Has The Greatest Electronegativity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
