What Reagents Are Necessary To Perform The Following Reaction
listenit
Mar 14, 2025 · 7 min read
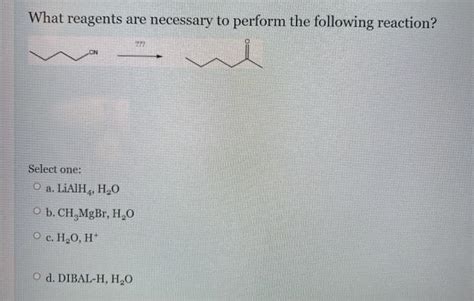
Table of Contents
What Reagents are Necessary to Perform the Following Reaction? A Comprehensive Guide
This article delves into the crucial aspect of reagent selection for chemical reactions. Choosing the right reagents is paramount for successful synthesis, ensuring high yield, selectivity, and minimizing unwanted byproducts. We will explore the factors influencing reagent choice, focusing on different reaction types and providing examples to illustrate the principles involved. This guide aims to be a comprehensive resource for both students and experienced chemists navigating the complexities of chemical synthesis.
Understanding the Importance of Reagent Selection
The success of any chemical reaction hinges critically on the selection of appropriate reagents. Reagents are substances that participate in a chemical reaction, transforming reactants into products. The choice of reagent is determined by several crucial factors:
-
Reaction type: Different reactions require different reagents. For example, an oxidation reaction will necessitate an oxidizing agent, while a reduction reaction will demand a reducing agent.
-
Substrate reactivity: The reactivity of the starting material (substrate) dictates the strength and type of reagent needed. A less reactive substrate might require a more potent reagent, whereas a highly reactive substrate might react readily with a milder reagent.
-
Selectivity: Ideally, a reagent should selectively react with the desired functional group without affecting other functional groups present in the molecule. This is crucial for complex molecules where multiple reactive sites exist.
-
Yield and purity: The chosen reagent should maximize product yield and minimize the formation of unwanted byproducts. This often involves considering reaction conditions like temperature, pressure, and solvent.
-
Cost and availability: While efficacy is paramount, practical considerations like cost and availability of the reagents play a vital role, especially in large-scale synthesis.
-
Toxicity and safety: The safety of the reagents and the potential environmental impact of the reaction are crucial factors in reagent selection. Health and safety protocols must always be strictly followed.
Categorizing Reagents Based on Reaction Types
Reagents can be broadly categorized based on the type of reaction they facilitate. Let's explore some common examples:
1. Oxidizing Agents: These reagents facilitate oxidation reactions, involving the loss of electrons or an increase in oxidation state.
-
Potassium permanganate (KMnO₄): A strong oxidizing agent used in various oxidations, including the oxidation of alcohols to ketones or carboxylic acids. It's known for its versatility but can be harsh under certain conditions.
-
Chromic acid (H₂CrO₄): Another powerful oxidizing agent often used for alcohol oxidation. However, it's highly toxic and carcinogenic, limiting its use in modern organic chemistry.
-
Jones reagent (CrO₃ in H₂SO₄): A safer and more controlled alternative to chromic acid, commonly used for oxidizing primary and secondary alcohols.
-
Pyridinium chlorochromate (PCC): A milder oxidizing agent compared to chromic acid, selectively oxidizing primary alcohols to aldehydes without further oxidation to carboxylic acids.
-
Dess-Martin periodinane (DMP): A highly selective and efficient oxidizing agent for alcohols, particularly useful for converting primary alcohols to aldehydes and secondary alcohols to ketones.
2. Reducing Agents: These reagents facilitate reduction reactions, involving the gain of electrons or a decrease in oxidation state.
-
Lithium aluminum hydride (LiAlH₄): A powerful reducing agent capable of reducing a wide range of functional groups, including esters, ketones, aldehydes, and carboxylic acids. It's highly reactive and requires careful handling.
-
Sodium borohydride (NaBH₄): A milder reducing agent compared to LiAlH₄, typically used for the reduction of aldehydes and ketones to alcohols. It's less reactive and safer to handle.
-
Diborane (B₂H₆): Used for the reduction of esters, ketones, aldehydes, and carboxylic acids. It is highly toxic and requires specialized handling.
-
Palladium on carbon (Pd/C): A heterogeneous catalyst often used in hydrogenation reactions, reducing alkenes and alkynes to alkanes.
3. Nucleophiles: These reagents possess a lone pair of electrons and are attracted to positively charged or electron-deficient centers (electrophiles).
-
Grignard reagents (RMgX): Organomagnesium halides that act as strong nucleophiles, adding to carbonyl groups to form alcohols.
-
Organolithium reagents (RLi): Similar to Grignard reagents, but generally more reactive.
-
Cyanide ion (CN⁻): A nucleophile used in nucleophilic addition reactions, often forming nitriles.
-
Alcohols (ROH): Can act as weak nucleophiles in certain reactions, particularly under acidic conditions.
4. Electrophiles: These reagents are electron-deficient and attracted to nucleophiles.
-
Alkyl halides (RX): Common electrophiles that undergo nucleophilic substitution reactions.
-
Acid chlorides (RCOCl): Highly reactive electrophiles often used in acylations.
-
Aldehydes and ketones: Can act as electrophiles in nucleophilic addition reactions.
5. Bases: These reagents abstract protons (H⁺) and increase the basicity of the reaction medium.
-
Sodium hydroxide (NaOH): A strong base used in various reactions, including saponification and base-catalyzed condensations.
-
Potassium hydroxide (KOH): Similar to NaOH but often preferred in specific applications.
-
Potassium tert-butoxide (t-BuOK): A strong, sterically hindered base frequently used in elimination reactions.
-
Lithium diisopropylamide (LDA): A very strong, non-nucleophilic base commonly used in enolate formation.
6. Acids: These reagents donate protons (H⁺) and increase the acidity of the reaction medium.
-
Sulfuric acid (H₂SO₄): A strong acid used in various reactions, including esterification and dehydration.
-
Hydrochloric acid (HCl): A strong acid used in many reactions, including acid-catalyzed additions.
-
Acetic acid (CH₃COOH): A weak acid often used as a solvent or catalyst.
7. Catalysts: These reagents increase the rate of a reaction without being consumed themselves.
-
Lewis acids (e.g., AlCl₃, BF₃): Accept electron pairs and activate electrophiles.
-
Transition metal catalysts (e.g., Pd, Pt, Rh): Used in a wide range of reactions, including cross-coupling reactions and hydrogenations.
-
Enzymes: Biological catalysts that accelerate specific reactions under mild conditions.
Factors Influencing Reagent Choice: A Case Study
Let's consider a specific example to illustrate the complexities involved in reagent selection. Suppose we want to synthesize an alcohol from a ketone. Several options exist, each with its own advantages and disadvantages:
-
Using NaBH₄: Sodium borohydride is a common reagent for ketone reduction. It's relatively mild, inexpensive, and easy to handle. However, it might not be suitable for sterically hindered ketones or those containing other reducible functional groups.
-
Using LiAlH₄: Lithium aluminum hydride is a much stronger reducing agent, capable of reducing even sterically hindered ketones. However, it's more reactive, requiring anhydrous conditions and careful handling. It’s also more expensive.
-
Using catalytic hydrogenation: Catalytic hydrogenation with a catalyst like Pd/C and H₂ is a gentler approach, but it can be slower and may require higher pressure.
The optimal choice depends on the specific ketone, the presence of other functional groups, the desired yield, and safety considerations. A careful assessment of these factors is necessary to select the most appropriate reagent.
Advanced Considerations in Reagent Selection
Beyond the basic categorization, several advanced considerations further refine reagent selection:
-
Stereochemistry: For reactions involving chiral centers, reagent selection must account for stereoselectivity – the preferential formation of one stereoisomer over others. Chiral reagents or catalysts can be employed to achieve high stereoselectivity.
-
Green chemistry principles: Increasingly, the environmental impact of reagents and reaction conditions is a primary concern. Green chemistry principles encourage the use of environmentally benign reagents and solvents, minimizing waste and promoting sustainability.
-
Reagent compatibility: The chosen reagents must be compatible with each other and the reaction conditions. Incompatible reagents can lead to unwanted side reactions or decomposition.
-
Scale-up: When scaling up a reaction from laboratory to industrial scale, reagent selection needs to take into account cost-effectiveness, availability, and safety considerations on a larger scale.
Conclusion
Choosing the right reagents is a critical step in chemical synthesis. This process involves a careful consideration of numerous factors, including reaction type, substrate reactivity, selectivity, yield, cost, safety, and environmental impact. Understanding these factors and employing a systematic approach to reagent selection is crucial for successful and efficient chemical synthesis. Continuous learning and exploration of the vast chemical literature remain essential for mastering this crucial aspect of organic chemistry and beyond. This article provides a foundational overview, and further research into specific reactions and reagents will enhance your expertise. Always prioritize safety and follow established laboratory protocols when handling chemicals.
Latest Posts
Latest Posts
-
Whats The Lcm Of 8 And 10
Mar 14, 2025
-
8 4u 1 12u 11 2u 6
Mar 14, 2025
-
Derivative Of Ln 1 X 2
Mar 14, 2025
-
A Square Is A Rectangle
Mar 14, 2025
-
Does A Trapezoid Have Right Angles
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Reagents Are Necessary To Perform The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
