What Is The Overall Charge Of The Nucleus
listenit
Mar 14, 2025 · 6 min read
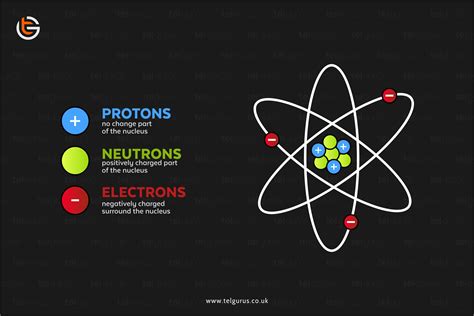
Table of Contents
What is the Overall Charge of the Nucleus?
The nucleus, residing at the heart of every atom, holds the key to understanding the atom's properties and its interactions with the world around it. A fundamental characteristic of the nucleus is its overall charge. This article delves deep into the nature of nuclear charge, explaining its origin, its impact on atomic structure and behavior, and its significance in various scientific fields.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before diving into the nuclear charge, let's establish a foundational understanding of atomic structure. Atoms consist of three primary subatomic particles:
- Protons: Positively charged particles located within the nucleus.
- Neutrons: Neutral particles (no charge) also residing in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or clouds.
The arrangement of these particles dictates the atom's overall properties. The number of protons, in particular, defines the element itself. This number is known as the atomic number.
The Source of Nuclear Charge: Protons
The overall charge of the nucleus is positive, and this positivity stems directly from the presence of protons. Each proton carries a single unit of positive charge, conventionally represented as +1. The number of protons in the nucleus, therefore, directly determines the magnitude of the positive nuclear charge.
For instance, a hydrogen atom (atomic number 1) possesses one proton in its nucleus, resulting in a nuclear charge of +1. A helium atom (atomic number 2) has two protons, leading to a nuclear charge of +2. This linear relationship between the number of protons and the nuclear charge is crucial for understanding various atomic and nuclear phenomena.
Isotopes and Nuclear Charge: A Deeper Dive
While the number of protons determines the element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. For example, carbon-12 and carbon-14 are isotopes of carbon, both having 6 protons but differing in the number of neutrons (6 and 8, respectively).
Crucially, the number of neutrons does not affect the overall charge of the nucleus. Neutrons are electrically neutral, meaning they contribute no charge to the nucleus. Therefore, isotopes of the same element have the identical nuclear charge despite their varying neutron counts. The change in neutron number affects the mass number (total number of protons and neutrons) but leaves the positive charge unchanged.
The Significance of Nuclear Charge: Its Influence on Atomic Behavior
The positive nuclear charge plays a pivotal role in shaping the atom's behavior and its interactions:
-
Electron Attraction: The positive charge of the nucleus exerts an attractive force on the negatively charged electrons, holding them in their orbits. This electrostatic attraction is the fundamental force responsible for the atom's stability. The strength of this attraction increases with increasing nuclear charge, leading to tighter electron orbits in atoms with higher atomic numbers.
-
Chemical Bonding: The interaction between the outermost electrons (valence electrons) and the nuclear charge governs how atoms form chemical bonds. Atoms tend to react in ways that achieve a stable electron configuration, often by gaining, losing, or sharing electrons. The positive nuclear charge influences the ability of an atom to attract or share electrons, directly impacting its chemical reactivity. Elements with high nuclear charge often form stronger bonds.
-
Ionization: The nuclear charge also affects the ease with which an atom can lose or gain electrons to form ions. Atoms with a high nuclear charge tend to hold onto their electrons more tightly, making them less likely to ionize. Conversely, atoms with lower nuclear charge are more prone to ionization.
Nuclear Charge and Periodic Trends
The periodic table's arrangement reflects the systematic variation in nuclear charge. As you move across a period (row) from left to right, the nuclear charge increases, leading to several predictable trends in atomic properties:
- Electronegativity: The tendency of an atom to attract electrons in a chemical bond increases with increasing nuclear charge across a period.
- Ionization Energy: The energy required to remove an electron from an atom increases with increasing nuclear charge.
- Atomic Radius: The size of an atom generally decreases across a period due to the stronger attraction between the nucleus and electrons.
These trends are directly attributable to the increasing positive charge of the nucleus as you traverse a period.
Beyond the Atom: Nuclear Charge in Larger Systems
The concept of nuclear charge extends beyond individual atoms to influence the behavior of larger systems:
-
Molecules: The interaction between the nuclei and electrons in different atoms forms the basis of chemical bonding in molecules. The arrangement of atoms within a molecule, and the resulting molecular geometry, are influenced by the individual nuclear charges.
-
Ionic Compounds: In ionic compounds, the transfer of electrons from one atom to another results in the formation of ions – charged particles. The magnitude of the charge on these ions is directly related to the nuclear charge of the constituent atoms.
-
Nuclear Reactions: Nuclear reactions, such as nuclear fusion and fission, involve changes within the nucleus itself. The nuclear charge plays a crucial role in determining the stability and reactivity of nuclei, influencing the energy released or absorbed during these reactions. The high nuclear charge in heavier elements contributes to their instability and susceptibility to radioactive decay.
Nuclear Charge and Isotopic Abundance
The stability of a nucleus is influenced by the balance between the number of protons and neutrons. Some isotopes are more stable than others, and this stability is reflected in their relative abundance in nature. The overall nuclear charge, determined by the number of protons, plays a significant role in this stability. Isotopes with a "magic number" of protons or neutrons (numbers associated with particularly stable nuclear configurations) tend to be more abundant.
Measuring Nuclear Charge: Techniques and Applications
Precisely measuring nuclear charge is essential in various scientific fields. While the number of protons (and therefore the nuclear charge) is usually readily available from the atomic number, experimental methods verify and refine our understanding:
-
X-ray spectroscopy: The energy of X-rays emitted by an atom is sensitive to the nuclear charge. Analyzing the X-ray spectrum allows scientists to determine the effective nuclear charge experienced by the inner electrons.
-
Electron diffraction: The scattering of electrons by an atom provides information about the distribution of charge within the atom, indirectly revealing information about the nuclear charge.
-
Nuclear magnetic resonance (NMR): NMR spectroscopy utilizes the magnetic properties of atomic nuclei, which are influenced by the nuclear charge, to study the structure and dynamics of molecules.
These techniques are indispensable in various fields, including materials science, chemistry, and nuclear physics.
Conclusion: A Fundamental Property with Far-Reaching Implications
The overall charge of the nucleus, a seemingly simple concept, holds immense significance in shaping the properties of matter. From the stability of individual atoms to the behavior of complex molecules and nuclear reactions, the positive charge of the nucleus exerts a profound influence. Understanding this fundamental property is crucial for comprehending the intricacies of the physical world and pushing the boundaries of scientific knowledge. The ongoing research into nuclear structure and reactions continuously refines our understanding of nuclear charge and its implications across various scientific disciplines. The consistent exploration and innovation in this field promises to reveal further fascinating insights into the very foundation of matter itself.
Latest Posts
Latest Posts
-
What Is 68 Of 118 Tons
Mar 15, 2025
-
Is Boiling Water A Physical Change Or A Chemical Change
Mar 15, 2025
-
Is It Better To Write Zn2 Or Zn 2
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Overall Charge Of The Nucleus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
