Which Change Of State Is Shown In The Model
listenit
Mar 15, 2025 · 6 min read
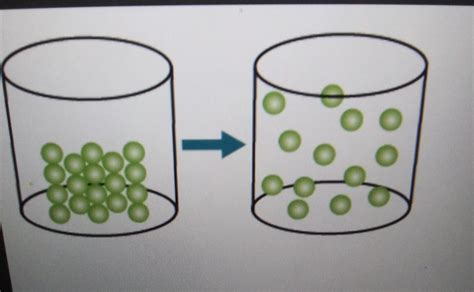
Table of Contents
Which Change of State is Shown in the Model? A Comprehensive Guide
Understanding changes of state is fundamental to grasping the nature of matter and its behavior under varying conditions. This comprehensive guide will delve into the various changes of state, explaining each one in detail and providing examples to help you identify which change of state is depicted in a given model or scenario. We'll explore the processes involved, the energy changes associated with them, and the practical applications of this knowledge.
Understanding States of Matter
Before we dive into the changes, let's briefly review the three primary states of matter:
-
Solid: Solids have a definite shape and volume. Their particles are tightly packed and have strong intermolecular forces holding them in a fixed arrangement. Think of ice, a rock, or a metal bar.
-
Liquid: Liquids have a definite volume but take the shape of their container. Their particles are closer together than in gases but further apart and more mobile than in solids. Water, oil, and juice are all examples of liquids.
-
Gas: Gases have neither a definite shape nor volume. Their particles are far apart and move freely, with weak intermolecular forces. Air, helium, and steam are examples of gases.
There is also a fourth state of matter, plasma, which is an ionized gas. However, for the purposes of this guide, we will focus on the three primary states.
The Six Changes of State
Matter can transition between these three states through six different processes:
1. Melting (Solid to Liquid)
Melting is the change of state from a solid to a liquid. This occurs when the thermal energy (heat) added to a solid overcomes the intermolecular forces holding its particles together. The particles gain kinetic energy, moving more rapidly and breaking free from their fixed positions. The melting point is the temperature at which a solid melts. Examples: Ice melting into water, chocolate melting, wax melting.
Keywords: melting, melting point, fusion, solid to liquid, heat absorption, endothermic.
2. Freezing (Liquid to Solid)
Freezing is the reverse of melting; it's the change of state from a liquid to a solid. This happens when thermal energy is removed from a liquid, causing its particles to slow down and the intermolecular forces to become stronger, forming a rigid structure. The freezing point is the temperature at which a liquid freezes. Importantly, the freezing point of a substance is generally the same as its melting point. Examples: Water freezing into ice, molten lava solidifying into rock, liquid metal solidifying in a mold.
Keywords: freezing, freezing point, solidification, liquid to solid, heat release, exothermic.
3. Vaporization (Liquid to Gas)
Vaporization is the change of state from a liquid to a gas. This can occur through two main processes:
-
Boiling: Boiling occurs when a liquid is heated to its boiling point, a temperature at which the vapor pressure of the liquid equals the external pressure. Bubbles of vapor form throughout the liquid and rise to the surface.
-
Evaporation: Evaporation is a slower process that occurs at the surface of a liquid at temperatures below the boiling point. Some of the more energetic particles at the surface have enough kinetic energy to escape into the gas phase.
Examples: Water boiling in a kettle, water evaporating from a puddle, perfume vaporizing into the air.
Keywords: vaporization, boiling, evaporation, boiling point, liquid to gas, heat absorption, endothermic.
4. Condensation (Gas to Liquid)
Condensation is the reverse of vaporization; it's the change of state from a gas to a liquid. This occurs when a gas cools down, causing its particles to lose kinetic energy and come closer together. The intermolecular forces become stronger, and the gas condenses into a liquid. Examples: Dew forming on grass, clouds forming in the sky, steam condensing on a mirror.
Keywords: condensation, gas to liquid, heat release, exothermic, dew point.
5. Sublimation (Solid to Gas)
Sublimation is the change of state from a solid directly to a gas, without passing through the liquid phase. This happens when the particles in a solid have enough kinetic energy to overcome the intermolecular forces and escape directly into the gas phase. This requires a significant amount of energy. Examples: Dry ice (solid carbon dioxide) turning into carbon dioxide gas, snow disappearing without melting (it sublimes), freeze-dried food.
Keywords: sublimation, solid to gas, heat absorption, endothermic.
6. Deposition (Gas to Solid)
Deposition is the reverse of sublimation; it's the change of state from a gas directly to a solid, without passing through the liquid phase. This occurs when gas particles lose enough kinetic energy to become tightly bound in a solid structure. Examples: Frost forming on a cold surface, snow forming in clouds (though this often involves several processes), the formation of ice crystals on windows in winter.
Keywords: deposition, gas to solid, heat release, exothermic.
Identifying the Change of State in a Model
When presented with a model depicting a change of state, carefully observe the following:
-
Initial and Final States: Identify the initial state of matter (solid, liquid, or gas) and the final state of matter. This will immediately narrow down the possibilities.
-
Energy Transfer: Determine whether heat is being added to the system (endothermic process) or removed from the system (exothermic process). Heating often leads to melting, vaporization, or sublimation, while cooling often leads to freezing, condensation, or deposition.
-
Particle Arrangement: Examine how the particles are arranged in the model. Are they tightly packed (solid), loosely packed (liquid), or far apart (gas)? The change in particle arrangement reflects the change of state.
-
Phase Diagram (if provided): A phase diagram shows the conditions of temperature and pressure under which a substance exists in different states. If provided, it can help you pinpoint the specific change of state.
Real-World Applications
Understanding changes of state is crucial in many areas, including:
-
Meteorology: Understanding condensation, evaporation, sublimation, and deposition is fundamental to predicting weather patterns and explaining phenomena like cloud formation, rain, snow, and frost.
-
Chemistry: Changes of state are essential in many chemical processes, such as purification, separation techniques (distillation), and the formation of crystals.
-
Materials Science: The properties of materials are often determined by their state and changes between states. For example, the process of melting and solidification is critical in the manufacturing of metals and plastics.
-
Food Science: Freezing, evaporation, sublimation, and other changes of state are important techniques used in food preservation, processing, and preparation.
-
Engineering: Understanding changes of state is crucial in designing systems involving fluids, such as heating and cooling systems, refrigeration, and power generation.
Conclusion
Changes of state are fascinating and essential processes that govern the behavior of matter. By understanding the characteristics of each change of state – melting, freezing, vaporization, condensation, sublimation, and deposition – you can better interpret models, predict outcomes, and appreciate the significance of these processes in our everyday lives and across various scientific and engineering disciplines. Remember to carefully observe the initial and final states, the energy transfer, and the particle arrangement to correctly identify the change of state depicted in any given model. This knowledge provides a fundamental building block for a deeper understanding of physics and chemistry.
Latest Posts
Latest Posts
-
P 10 P 7 8 9
Mar 15, 2025
-
How To Get Rid Of A Fraction
Mar 15, 2025
-
Number Of Valence Electrons Of Sodium
Mar 15, 2025
-
How Many Sigma And Pi Bonds In A Triple Bond
Mar 15, 2025
-
Why Does Active Transport Need Energy
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Which Change Of State Is Shown In The Model . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
