Number Of Valence Electrons Of Sodium
listenit
Mar 15, 2025 · 5 min read
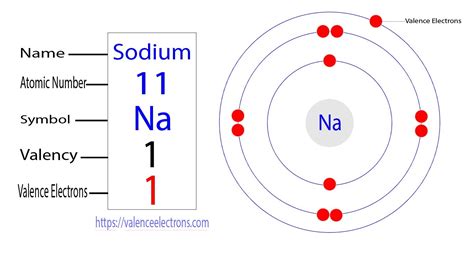
Table of Contents
The Number of Valence Electrons in Sodium: A Deep Dive
Sodium (Na), a ubiquitous element found in table salt and essential for life, holds a significant place in chemistry. Understanding its electronic structure, particularly the number of valence electrons, is crucial to comprehending its reactivity and behavior in various chemical reactions. This article will delve into the details of sodium's valence electrons, explaining its significance, how to determine it, and its implications in chemical bonding and properties.
Understanding Valence Electrons
Before focusing specifically on sodium, let's establish a clear understanding of valence electrons. These are the electrons located in the outermost shell, or energy level, of an atom. They are the electrons most involved in chemical bonding because they are farthest from the nucleus and experience the weakest attraction to it. The number of valence electrons largely determines an element's chemical properties and how it will interact with other elements. Atoms tend to gain, lose, or share valence electrons to achieve a stable electron configuration, often resembling a noble gas with a full outer shell (usually eight electrons, the octet rule).
Determining Sodium's Valence Electrons
Sodium's atomic number is 11, meaning a neutral sodium atom has 11 protons and 11 electrons. To determine the number of valence electrons, we need to examine its electron configuration. This configuration shows how the electrons are distributed among different energy levels or shells.
The electron configuration of sodium is 1s²2s²2p⁶3s¹.
Let's break this down:
- 1s²: This signifies two electrons in the first energy level (n=1), filling the 1s orbital.
- 2s²: Two electrons are in the second energy level (n=2), occupying the 2s orbital.
- 2p⁶: Six electrons populate the 2p orbitals within the second energy level.
- 3s¹: One electron resides in the 3s orbital of the third energy level (n=3).
The outermost shell for sodium is the third energy level (n=3), which contains only one electron in the 3s orbital. Therefore, sodium has one valence electron.
Visualizing Sodium's Electron Configuration
Imagine the electrons orbiting the sodium nucleus in distinct shells. The first shell can hold a maximum of two electrons, the second shell can hold up to eight, and so on. Sodium's electron configuration visually shows two electrons in the inner shell, eight in the second shell, and a single electron in the outermost shell. It's this lone electron in the outermost shell that defines its valence electron count.
Sodium's Reactivity and the Valence Electron
The presence of only one valence electron significantly impacts sodium's chemical reactivity. Atoms strive for stability, often achieved by having a full outer electron shell. Sodium readily loses its single valence electron to achieve the stable electron configuration of neon (1s²2s²2p⁶), which has a full outer shell of eight electrons. This electron loss forms a positively charged sodium ion (Na⁺).
This tendency to lose an electron makes sodium highly reactive, particularly with nonmetals that readily accept electrons. The strong electrostatic attraction between the positively charged sodium ion and the negatively charged nonmetal ion forms ionic bonds, resulting in the formation of ionic compounds.
Examples of Sodium's Reactivity
-
Reaction with Chlorine: Sodium reacts vigorously with chlorine gas (Cl₂), forming sodium chloride (NaCl), or common table salt. Sodium loses its valence electron to chlorine, which gains it, forming Na⁺ and Cl⁻ ions held together by ionic bonds.
-
Reaction with Water: Sodium reacts violently with water, producing hydrogen gas (H₂) and sodium hydroxide (NaOH). The single valence electron is transferred to water molecules, leading to the formation of hydrogen gas and a highly alkaline solution.
Implications of Sodium's Valence Electron
The single valence electron is responsible for numerous properties and behaviors of sodium:
-
Low Ionization Energy: The energy required to remove the single valence electron from sodium is relatively low, reflecting its tendency to lose an electron and form a stable ion. This low ionization energy contributes to its high reactivity.
-
Metallic Character: Sodium exhibits strong metallic characteristics, such as high electrical and thermal conductivity. This is due to the delocalized valence electrons forming a "sea" of electrons that can move freely throughout the metallic structure, facilitating the transfer of both heat and electricity.
-
Low Electronegativity: Sodium has a low electronegativity, meaning it has a weak tendency to attract electrons in a chemical bond. This aligns with its preference to lose its valence electron rather than gain more.
-
Formation of Ionic Compounds: As previously discussed, sodium's propensity to lose its valence electron leads to the formation of ionic compounds, where strong electrostatic forces hold the oppositely charged ions together.
Sodium's Role in Biological Systems
Sodium plays a crucial role in various biological systems. Its single valence electron facilitates its ability to form ions (Na⁺) which are vital for:
-
Nerve Impulse Transmission: Sodium ions are critical for the propagation of nerve impulses throughout the nervous system. The movement of Na⁺ ions across cell membranes generates electrical signals that enable communication between nerve cells.
-
Muscle Contraction: Similar to nerve impulse transmission, sodium ions participate in muscle contraction. Changes in the concentration of sodium ions within muscle cells trigger the contractile processes necessary for movement.
-
Fluid Balance: Sodium ions contribute to the regulation of fluid balance within the body. They help maintain the osmotic pressure that controls the distribution of water between different compartments within the body.
Conclusion: The Significance of One Electron
While seemingly insignificant on its own, the single valence electron of sodium is the key to understanding its reactivity, its physical properties, and its vital role in biological systems. This one electron determines how sodium interacts with other elements, forms compounds, and participates in essential life processes. Studying sodium's valence electron provides a fundamental understanding of atomic structure, chemical bonding, and the broader implications of electronic configuration in determining the properties of matter. This simple concept underpins a vast array of chemical and biological phenomena. Understanding the significance of sodium's single valence electron is essential for anyone studying chemistry, biology, or related fields.
Latest Posts
Latest Posts
-
Proteins Are Made Of Monomers Called
Mar 15, 2025
-
X 2 4 X 2 Graph
Mar 15, 2025
-
What Is The Difference Between Ion And Atom
Mar 15, 2025
-
What Is 2 3 Of 10
Mar 15, 2025
-
1 1 3 Cups Divided By 2
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons Of Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
