What Is The Difference Between Ion And Atom
listenit
Mar 15, 2025 · 6 min read
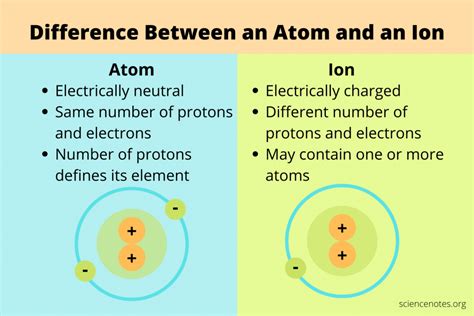
Table of Contents
What's the Difference Between Ions and Atoms? A Deep Dive into Atomic Structure and Electrical Charge
Understanding the fundamental building blocks of matter is crucial for grasping many scientific concepts. While the terms "atom" and "ion" are often used interchangeably in casual conversation, they represent distinct states of matter with key differences in their structure and properties. This comprehensive guide will explore the nuances separating atoms and ions, delving into their electronic configurations, chemical behavior, and significance in various scientific fields.
Atoms: The Fundamental Units of Matter
Atoms are the basic units of matter that retain the chemical properties of an element. They are incredibly small, typically measuring only about 0.1 to 0.5 nanometers in diameter. Each atom is composed of three fundamental subatomic particles:
1. Protons: Positively Charged Particles
Protons reside in the atom's nucleus, a dense central region. Their positive charge (+1) is crucial in determining an element's atomic number and its place on the periodic table. The number of protons defines the element; for example, all atoms with one proton are hydrogen, while those with six protons are carbon.
2. Neutrons: Neutral Particles
Neutrons, also located in the nucleus, have no electrical charge (0). Their presence contributes to an atom's mass but not its chemical properties. Isotopes of an element are atoms with the same number of protons but a different number of neutrons, resulting in variations in mass.
3. Electrons: Negatively Charged Particles
Electrons occupy the space surrounding the nucleus in regions called electron shells or orbitals. These shells represent different energy levels, with electrons in lower shells being closer to the nucleus and having lower energy. The number of electrons typically equals the number of protons in a neutral atom, resulting in a net charge of zero. Electrons are responsible for chemical bonding and interactions between atoms.
Ions: Atoms with an Electrical Charge
Unlike atoms, which are electrically neutral, ions carry a net electrical charge. This charge arises from an imbalance in the number of protons and electrons within the atom.
Ion Formation: Gaining or Losing Electrons
Ions are formed through the gain or loss of electrons from a neutral atom. This process is called ionization.
-
Cations: When an atom loses one or more electrons, it becomes positively charged because the number of protons now exceeds the number of electrons. These positively charged ions are called cations. Metals, with their relatively low electronegativity, tend to form cations. For example, a sodium atom (Na) can lose one electron to become a sodium cation (Na⁺).
-
Anions: Conversely, when an atom gains one or more electrons, it becomes negatively charged because the number of electrons now surpasses the number of protons. These negatively charged ions are called anions. Nonmetals, with their higher electronegativity, tend to form anions. For example, a chlorine atom (Cl) can gain one electron to become a chloride anion (Cl⁻).
The Role of Electron Shells in Ion Formation
The stability of an atom's electron configuration significantly influences its ability to form ions. Atoms tend to gain or lose electrons to achieve a stable electron configuration, often resembling that of a noble gas (Group 18 elements). This stable configuration, usually with a full outer electron shell (octet rule), makes the ion less reactive.
Key Differences Between Atoms and Ions: A Summary Table
| Feature | Atom | Ion |
|---|---|---|
| Electrical Charge | Neutral (0) | Positive (cation) or Negative (anion) |
| Electron Number | Equal to proton number | Unequal to proton number |
| Formation | Naturally occurring fundamental unit | Formed through ionization (gain/loss of electrons) |
| Chemical Reactivity | Varies, depends on electron configuration | Generally more reactive than the parent atom |
| Examples | Hydrogen (H), Oxygen (O), Carbon (C) | Sodium ion (Na⁺), Chloride ion (Cl⁻), Sulfate ion (SO₄²⁻) |
The Significance of Ions in Chemistry and Biology
Ions play a crucial role in a vast array of chemical and biological processes:
1. Ionic Compounds: The Foundation of Many Materials
Ions are fundamental components of ionic compounds, formed through the electrostatic attraction between oppositely charged ions. These compounds are essential building blocks of many materials, including salts, minerals, and many inorganic substances. Examples include sodium chloride (NaCl, table salt), calcium carbonate (CaCO₃, limestone), and many others.
2. Electrolytes: Conducting Electricity in Solutions
Ions dissolved in a solution can conduct electricity. These solutions are called electrolytes and are crucial in various applications, including batteries, electrochemical cells, and biological systems. Electrolytes in our bodies, such as sodium, potassium, and chloride ions, are essential for nerve impulse transmission, muscle contraction, and fluid balance.
3. Chemical Reactions: Driving Force of Many Processes
Ions participate in a wide range of chemical reactions, including acid-base reactions, redox reactions, and precipitation reactions. Their charges and ability to interact with other ions and molecules make them key players in chemical transformations.
4. Biological Processes: Essential for Life
Ions are essential for many biological processes. For example, calcium ions (Ca²⁺) are crucial for muscle contraction and bone formation, while sodium (Na⁺) and potassium (K⁺) ions are vital for nerve impulse transmission and maintaining cell membrane potential. The proper balance of ions within cells and body fluids is critical for life.
Advanced Concepts: Isotopes and Isobars
While the primary focus has been on the difference between atoms and ions, understanding isotopes and isobars adds further depth to atomic structure.
-
Isotopes: Atoms of the same element (same number of protons) but with a different number of neutrons are called isotopes. For example, carbon-12 (⁶¹²C) and carbon-14 (⁶¹⁴C) are isotopes of carbon, differing only in the number of neutrons (6 and 8 respectively). Isotopes can have different stabilities, with some being radioactive (like carbon-14) while others are stable.
-
Isobars: Isobars are atoms of different elements (different number of protons) that have the same mass number (total number of protons and neutrons). For example, ¹⁴C (carbon-14) and ¹⁴N (nitrogen-14) are isobars.
Conclusion: A Fundamental Distinction
The distinction between atoms and ions is a fundamental one in chemistry and related sciences. While both are composed of protons, neutrons, and electrons, their electrical charge and chemical behavior differ significantly. Atoms are neutral and serve as the basic building blocks of elements, while ions are charged particles formed through the gain or loss of electrons. Understanding these differences is essential for grasping the complexities of chemical bonding, reactions, and their crucial role in biological and material science. From the formation of ionic compounds to the regulation of biological processes, ions play a critical role in shaping the world around us. By exploring the intricate details of atomic structure and charge, we gain a deeper understanding of the fundamental principles that govern the behavior of matter.
Latest Posts
Latest Posts
-
12 Is What Percent Of 20
Mar 15, 2025
-
Creatine Phosphate Functions Within The Muscle Cells By
Mar 15, 2025
-
What Is 2 And 2 5 As A Decimal
Mar 15, 2025
-
What Elements In The Same Period Have In Common
Mar 15, 2025
-
Common Factors Of 36 And 54
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Ion And Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
