What Elements In The Same Period Have In Common
listenit
Mar 15, 2025 · 5 min read
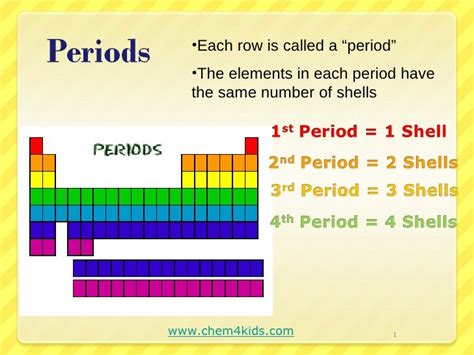
Table of Contents
What Elements in the Same Period Have in Common: A Deep Dive into Periodic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding the relationships between elements, particularly those within the same period (horizontal row), is crucial for predicting their behavior and reactivity. This article delves into the commonalities of elements sharing a period, exploring the underlying reasons for these similarities and differences.
The Significance of Periods in the Periodic Table
Elements within the same period share the same highest principal energy level (n) for their valence electrons. This shared principal quantum number is the defining characteristic that dictates many of their properties. The number of electron shells also remains constant across a period, while the number of electrons and protons steadily increase from left to right. This systematic increase profoundly impacts the elements' atomic radius, ionization energy, electronegativity, and metallic character.
Understanding Valence Electrons and their Role
Valence electrons, the electrons in the outermost shell, are the key players in determining an element's chemical behavior. They are responsible for forming chemical bonds with other atoms. Elements within the same period have their valence electrons in the same principal energy level, leading to similar bonding patterns and chemical reactivity, although the number of valence electrons differs across the period. This is why elements in the same period exhibit a range of properties, even while sharing a fundamental commonality.
Key Periodic Trends Across a Period
Several key properties exhibit systematic trends across a period, reflecting the increasing nuclear charge and the gradual filling of the outermost electron shell.
1. Atomic Radius: A Steady Decrease
Moving from left to right across a period, the atomic radius generally decreases. This is because the number of protons in the nucleus increases, resulting in a stronger positive charge attracting the electrons more tightly. While another electron shell is added, the increased nuclear charge outweighs the effect of the added shell, leading to a smaller atomic size. This trend is consistent across all periods, though the magnitude of the decrease can vary.
2. Ionization Energy: The Energy to Remove an Electron
Ionization energy, the energy required to remove an electron from a neutral atom, generally increases across a period. This is a direct consequence of the increasing nuclear charge. The stronger attraction between the nucleus and the electrons makes it more difficult to remove an electron, requiring a greater input of energy. The exceptions to this trend are usually related to electron configurations involving half-filled or fully-filled subshells, which exhibit enhanced stability.
3. Electronegativity: The Tendency to Attract Electrons
Electronegativity, the ability of an atom to attract electrons in a chemical bond, also generally increases across a period. This is linked directly to the increasing nuclear charge. Atoms with higher electronegativity tend to pull electrons closer to themselves in a bond, leading to more polar bonds. The most electronegative elements reside on the right side of the periodic table (excluding the noble gases).
4. Metallic Character: A Gradual Shift to Non-metals
Metallic character, the tendency of an element to exhibit metallic properties like conductivity and malleability, generally decreases across a period. Elements on the left side of the period tend to be metals, while those on the right are nonmetals. This trend is connected to the ionization energy and electronegativity. Metals readily lose electrons, having low ionization energies and low electronegativities, while nonmetals tend to gain electrons, possessing high ionization energies and high electronegativities. The transition between metallic and nonmetallic character is gradual, with metalloids occupying an intermediate position.
Specific Examples: Comparing Periods
Let's analyze specific periods to illustrate these trends:
Period 2 (Li, Be, B, C, N, O, F, Ne)
- Lithium (Li) is an alkali metal, highly reactive, with a low ionization energy and electronegativity, and a large atomic radius for its period.
- Beryllium (Be) is an alkaline earth metal, less reactive than lithium, showing an increased ionization energy and electronegativity, and a smaller atomic radius.
- Boron (B) is a metalloid, exhibiting properties of both metals and nonmetals. The trend of decreasing atomic radius and increasing ionization energy and electronegativity continues.
- Carbon (C), Nitrogen (N), Oxygen (O), and Fluorine (F) are nonmetals, with increasing ionization energy and electronegativity and decreasing atomic radii.
- Neon (Ne) is a noble gas, extremely unreactive due to its complete valence electron shell.
Period 3 (Na, Mg, Al, Si, P, S, Cl, Ar)
Period 3 follows the same trends as Period 2. Sodium (Na) is an alkali metal, similar to Li, while Chlorine (Cl) is a halogen, similar to F, showcasing the repetition of properties within groups and the trends within periods. The differences in reactivity and other properties are a result of the increased nuclear charge and the slightly larger atomic radii compared to their Period 2 counterparts.
Exceptions and Irregularities
While the general trends described are consistent, there are exceptions and irregularities. These often stem from electron configurations with half-filled or fully-filled subshells, which offer enhanced stability. For instance, the ionization energy of nitrogen is slightly higher than oxygen, despite the higher nuclear charge of oxygen. This is because nitrogen has a half-filled p subshell, making it more stable and requiring more energy to remove an electron.
Conclusion: Predicting Properties from Periodic Position
Understanding the commonalities among elements within the same period is crucial for making predictions about their chemical and physical properties. The systematic trends in atomic radius, ionization energy, electronegativity, and metallic character are directly linked to the increasing nuclear charge and the filling of the outermost electron shell. While exceptions exist, the overarching principles provide a powerful framework for understanding and predicting the behavior of elements based solely on their position in the periodic table. This knowledge forms the foundation for numerous applications in chemistry, materials science, and related fields. Further exploration into specific groups and the nuances of electron configuration will deepen your understanding of the periodic table's intricate structure and its predictive power. By mastering these concepts, you gain the ability to interpret and predict the behavior of matter at a fundamental level.
Latest Posts
Latest Posts
-
What Is The Difference Between A Community And An Ecosystem
Mar 15, 2025
-
12 Cups Equals How Many Quarts
Mar 15, 2025
-
Which Wave Is Carrying The Most Energy
Mar 15, 2025
-
What Is The Gcf Of 36 And 54
Mar 15, 2025
-
X 3 3x 2 X 3 Factorise
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Elements In The Same Period Have In Common . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
