Proteins Are Made Of Monomers Called
listenit
Mar 15, 2025 · 7 min read
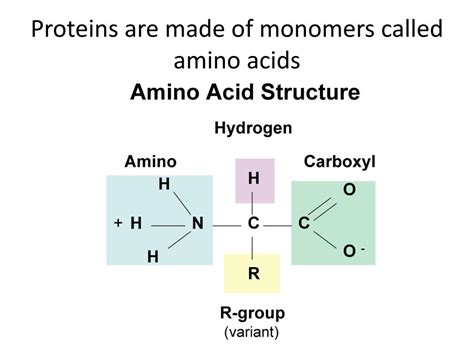
Table of Contents
Proteins Are Made of Monomers Called Amino Acids: A Deep Dive
Proteins are the workhorses of the cell, carrying out a vast array of functions crucial for life. From catalyzing biochemical reactions as enzymes to providing structural support as components of hair and nails, proteins' diverse roles stem from their intricate structures. But what are these complex molecules fundamentally made of? The answer lies in their building blocks: amino acids, the monomers that combine to form these essential biopolymers.
Understanding the Building Blocks: Amino Acids
Amino acids are organic molecules characterized by a central carbon atom (the alpha carbon) bonded to four groups:
- An amino group (-NH₂): This group is basic and acts as a proton acceptor.
- A carboxyl group (-COOH): This group is acidic and acts as a proton donor.
- A hydrogen atom (-H): A simple hydrogen atom.
- A variable side chain (R-group): This is the unique part of each amino acid, determining its properties and influencing the protein's overall structure and function.
This consistent core structure allows amino acids to link together through a process called peptide bond formation.
The 20 Standard Amino Acids
There are 20 standard amino acids used by the cellular machinery to build proteins. These amino acids are categorized based on their side chain properties:
-
Nonpolar, aliphatic amino acids: These amino acids have hydrocarbon side chains, making them hydrophobic (water-repelling). Examples include glycine, alanine, valine, leucine, isoleucine, and methionine. Their hydrophobic nature often places them in the interior of proteins, away from the aqueous environment of the cell.
-
Aromatic amino acids: These amino acids possess aromatic rings in their side chains, contributing to their hydrophobic nature. Phenylalanine, tyrosine, and tryptophan belong to this group. Their ability to absorb ultraviolet light is used in protein analysis techniques.
-
Polar, uncharged amino acids: These amino acids have side chains that are polar but do not carry a net charge at physiological pH. They are hydrophilic (water-attracting) and are often found on the protein's surface, interacting with the surrounding water molecules. Serine, threonine, cysteine, asparagine, and glutamine are examples. Cysteine is unique due to its sulfhydryl group (-SH), which can form disulfide bonds, crucial for protein stability.
-
Positively charged amino acids (basic amino acids): These amino acids have side chains with a positive charge at physiological pH. Lysine, arginine, and histidine fall into this category. Their positive charges participate in ionic interactions and contribute to protein-protein interactions.
-
Negatively charged amino acids (acidic amino acids): These amino acids possess side chains with a negative charge at physiological pH. Aspartic acid and glutamic acid are examples. Their negative charges also participate in ionic interactions, influencing protein structure and function.
The unique properties of each amino acid's side chain dictate its role in protein folding and function. A protein's amino acid sequence, determined by its gene, dictates its three-dimensional structure, which, in turn, determines its function.
Peptide Bond Formation: Linking Amino Acids
Amino acids link together to form proteins via peptide bonds. This is a covalent bond formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH₂) of another amino acid. This reaction releases a water molecule (H₂O), a process known as a dehydration reaction.
The resulting chain of amino acids is called a polypeptide. A protein can consist of one or more polypeptide chains. The sequence of amino acids in a polypeptide chain is referred to as its primary structure. This sequence is dictated by the genetic code.
Understanding Peptide Bond Characteristics
Peptide bonds possess several key characteristics:
-
Planar: The peptide bond exhibits partial double bond character due to resonance, restricting rotation around the bond and influencing protein conformation.
-
Trans configuration: The amino acid side chains typically lie on opposite sides of the peptide bond (trans configuration), maximizing stability.
-
Polar: The peptide bond is polar due to the electronegativity difference between oxygen and nitrogen, contributing to hydrogen bonding interactions within the protein.
From Primary to Quaternary Structure: Protein Folding
The linear sequence of amino acids (primary structure) is just the beginning of the protein's story. The polypeptide chain folds into a complex three-dimensional structure, driven by various interactions between amino acid side chains:
-
Secondary structure: This refers to local folding patterns within the polypeptide chain, primarily stabilized by hydrogen bonds between the backbone amide and carbonyl groups. Common secondary structures include alpha-helices and beta-sheets. Alpha-helices are coiled structures stabilized by intrachain hydrogen bonds, while beta-sheets are formed by hydrogen bonds between parallel or antiparallel polypeptide segments.
-
Tertiary structure: This refers to the overall three-dimensional arrangement of a single polypeptide chain. It is stabilized by a variety of interactions including hydrogen bonds, ionic bonds, hydrophobic interactions, disulfide bonds (between cysteine residues), and van der Waals forces. The tertiary structure is crucial for protein function. The precise folding is often influenced by chaperone proteins that assist in preventing aggregation and promoting correct folding.
-
Quaternary structure: This refers to the arrangement of multiple polypeptide chains (subunits) to form a functional protein complex. Hemoglobin, for example, consists of four polypeptide subunits. These interactions are similarly stabilized by the same forces as tertiary structures.
The Importance of Protein Structure and Function
The precise three-dimensional structure of a protein is directly linked to its function. Even a small change in the amino acid sequence can drastically alter the protein's structure and, consequently, its function. This is why mutations in genes encoding proteins can lead to various diseases.
For example:
-
Enzymes: The active site of an enzyme, the region where it binds to its substrate, is precisely formed by the tertiary structure. Any alteration in this structure can affect the enzyme's catalytic activity.
-
Structural proteins: Proteins like collagen, a major component of connective tissue, have a specific triple-helical structure crucial for its strength and elasticity.
-
Transport proteins: Membrane proteins involved in transporting molecules across cell membranes have specific structures that create channels or binding sites for their substrates.
-
Regulatory proteins: Many proteins act as regulators of gene expression or cellular processes, often by binding to specific DNA sequences or other proteins. Their precise structure is critical for these interactions.
Protein Synthesis: From Gene to Protein
The information for building a protein is encoded in the cell's DNA. This information is transcribed into messenger RNA (mRNA), which then travels to the ribosome, the protein synthesis machinery. At the ribosome, the mRNA sequence is translated into a polypeptide chain, with each three-nucleotide codon specifying a particular amino acid. This process is remarkably precise, ensuring that the correct amino acid is added to the growing polypeptide chain in the correct order.
The newly synthesized polypeptide chain then undergoes folding and modifications to achieve its functional three-dimensional structure. These modifications can include glycosylation (addition of sugar groups), phosphorylation (addition of phosphate groups), or proteolytic cleavage (cutting of the polypeptide chain). These post-translational modifications are crucial for protein function and regulation.
Conclusion: The Interplay of Amino Acids and Protein Function
Proteins are complex and versatile molecules, essential for virtually every aspect of life. Their remarkable abilities stem from their intricate structures, which are ultimately determined by the sequence of amino acids. Understanding the properties of each amino acid, how they link to form polypeptides, and the forces that drive protein folding is fundamental to comprehending the diverse roles of proteins in biological systems. From catalyzing biochemical reactions to providing structural support, proteins' functions are tightly coupled to their amino acid composition and three-dimensional structure. The remarkable precision of protein synthesis and folding highlights the elegant complexity of life itself. The study of proteins and their constituent amino acids continues to be a vibrant field, with ongoing research revealing new insights into protein structure, function, and their roles in health and disease.
Latest Posts
Latest Posts
-
How Many Sides Is A Dodecagon
Mar 15, 2025
-
Which Element Has The Lowest Ionization Energy
Mar 15, 2025
-
What Is The Lowest Common Multiple Of 9 And 15
Mar 15, 2025
-
Lcm Of 5 3 And 4
Mar 15, 2025
-
20 As A Percent Of 50
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Proteins Are Made Of Monomers Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
