Which Element Has The Lowest Ionization Energy
listenit
Mar 15, 2025 · 6 min read
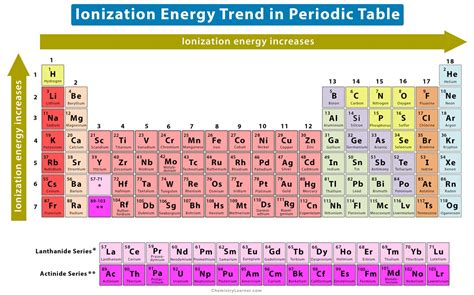
Table of Contents
Which Element Has the Lowest Ionization Energy? Unveiling the Secrets of Francium
The quest to identify the element with the lowest ionization energy leads us on a fascinating journey through the periodic table, exploring the fundamental principles of atomic structure and electron behavior. Ionization energy, a cornerstone concept in chemistry and physics, measures the energy required to remove an electron from a gaseous atom or ion. Understanding this property is crucial to predicting chemical reactivity and interpreting various atomic phenomena. This comprehensive exploration will not only pinpoint the element with the lowest ionization energy but also delve into the underlying reasons behind this property, clarifying the connection between atomic structure and ionization energy.
Understanding Ionization Energy: A Foundation
Before we delve into identifying the element with the lowest ionization energy, let's establish a solid understanding of the concept itself. Ionization energy, also known as ionization potential, is the minimum energy needed to remove the most loosely bound electron from a neutral gaseous atom in its ground state. This process results in the formation of a positively charged ion (cation). The energy required varies depending on the element and the specific electron being removed.
The first ionization energy (IE₁) refers to the removal of the first electron, the second ionization energy (IE₂) refers to the removal of the second electron, and so on. It's important to note that subsequent ionization energies are always higher than the preceding ones because removing an electron increases the effective nuclear charge experienced by the remaining electrons, making them harder to remove.
Factors Influencing Ionization Energy
Several factors govern an element's ionization energy:
-
Nuclear Charge: A higher nuclear charge exerts a stronger attractive force on the electrons, increasing the ionization energy. Elements with more protons in their nuclei require more energy to remove electrons.
-
Atomic Radius: A larger atomic radius means the outermost electrons are farther from the nucleus and experience a weaker attractive force. This results in lower ionization energy.
-
Shielding Effect: Inner electrons shield the outer electrons from the full positive charge of the nucleus. Increased shielding reduces the effective nuclear charge experienced by the outer electrons, lowering the ionization energy.
-
Electron Configuration: Elements with completely filled or half-filled subshells have increased stability, resulting in higher ionization energies. Conversely, elements with electrons in partially filled subshells have lower ionization energies.
The Alkali Metals: Contenders for the Lowest Ionization Energy
The elements with the lowest ionization energies reside in Group 1 of the periodic table – the alkali metals. These elements possess a single valence electron in their outermost shell, which is relatively far from the nucleus and experiences significant shielding from inner electrons. This combination of factors makes it relatively easy to remove this single electron, resulting in low ionization energies.
Comparing Alkali Metals: A Closer Look
Let's examine the alkali metals to determine which possesses the lowest ionization energy:
-
Lithium (Li): Possesses a relatively small atomic radius and moderate shielding, leading to a relatively higher ionization energy among the alkali metals.
-
Sodium (Na): Larger atomic radius and increased shielding compared to lithium, resulting in a lower ionization energy than lithium.
-
Potassium (K): Even larger atomic radius and stronger shielding compared to sodium, resulting in an even lower ionization energy.
-
Rubidium (Rb): The trend continues with rubidium exhibiting a larger atomic radius and stronger shielding compared to potassium. Its ionization energy is lower than potassium's.
-
Cesium (Cs): Cesium follows the pattern with an even larger atomic radius and increased shielding, resulting in a lower ionization energy than rubidium.
-
Francium (Fr): Francium, being the heaviest alkali metal, holds the lowest ionization energy. Its exceptionally large atomic radius and significant shielding effect minimize the attractive force of the nucleus on its single valence electron. This makes it significantly easier to remove this electron, leading to the lowest ionization energy among all elements.
Francium: The Champion of Low Ionization Energy
Based on the trends observed across the alkali metals, francium (Fr) undoubtedly holds the title of the element with the lowest ionization energy. This is a direct consequence of its position at the bottom of Group 1 in the periodic table. The sheer size of the francium atom and the significant shielding provided by its many inner electrons greatly reduce the effective nuclear charge experienced by its lone valence electron.
The Challenges of Studying Francium
While francium's position as the element with the lowest ionization energy is firmly established, studying its properties presents significant challenges. Francium is an extremely rare and radioactive element, with its most stable isotope having a half-life of only 22 minutes. This short half-life makes it extremely difficult to obtain and study in sufficient quantities to conduct detailed experiments and accurately measure its ionization energy directly. Most data about francium's properties are derived through theoretical calculations and extrapolation based on trends observed in other alkali metals.
However, these theoretical calculations and extrapolations strongly support the conclusion that francium has the lowest ionization energy. The consistent trend of decreasing ionization energy as we move down Group 1, coupled with francium's exceptionally large atomic radius and substantial shielding, leaves little doubt about its status as the champion of low ionization energy.
Beyond Francium: Extrapolation and Future Research
While francium currently holds the record, it’s important to consider the possibility of undiscovered elements with even lower ionization energies. Extrapolation based on periodic trends suggests that superheavy elements beyond francium, if they were to exist in a stable state for sufficient time, could potentially exhibit even lower ionization energies. However, the synthesis and study of these superheavy elements remain extremely challenging endeavors.
Future research focusing on advanced theoretical calculations and perhaps novel experimental techniques could provide a deeper understanding of ionization energy in these hypothetical superheavy elements. Moreover, exploring the behavior of francium under different conditions could yield valuable insights into the factors that influence its extremely low ionization energy.
Conclusion: Understanding the Significance
The identification of francium as the element with the lowest ionization energy underscores the intricate relationship between atomic structure and chemical behavior. The ability to predict and understand ionization energy is fundamental to our comprehension of chemical reactivity, bonding, and various other chemical phenomena. The challenges posed by francium's rarity and radioactivity highlight the ongoing need for refined theoretical models and innovative experimental methods in the study of chemical properties. This continuous exploration expands our fundamental knowledge of the atomic world, driving innovation in various scientific fields. While francium may hold the title today, the pursuit of understanding ionization energy continues, pushing the boundaries of our knowledge and uncovering the remarkable intricacies of the elements that make up our universe.
Latest Posts
Latest Posts
-
Which Has More Inertia Tennis Ball Or Basketball
Mar 17, 2025
-
Is Air An Element Or Compound Or Mixture
Mar 17, 2025
-
What Percent Is 15 Of 75
Mar 17, 2025
-
Y 2 X 1 2 1 Graph
Mar 17, 2025
-
How Many Years Are In 1000 Days
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Element Has The Lowest Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
