Is Air An Element Or Compound Or Mixture
listenit
Mar 17, 2025 · 6 min read
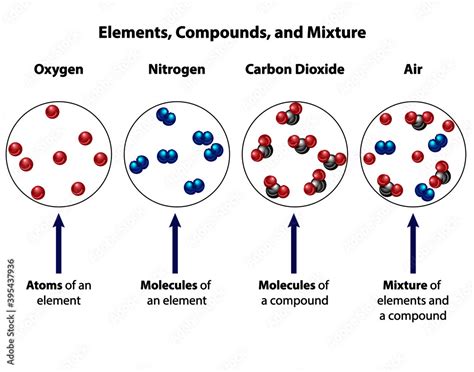
Table of Contents
Is Air an Element, Compound, or Mixture? Unveiling the Composition of Our Atmosphere
The air we breathe, the very essence that sustains life on Earth, is a topic of fundamental importance in science. But what exactly is air? Is it an element, a compound, or a mixture? This seemingly simple question opens a fascinating door to understanding the composition of our atmosphere and the fundamental building blocks of matter. The short answer is that air is a mixture. However, understanding why it's a mixture, and what constitutes that mixture, requires a deeper dive into the world of chemistry.
Understanding the Basic Classifications of Matter
Before we delve into the specifics of air, let's establish a clear understanding of the key terms: element, compound, and mixture.
Element: The Fundamental Building Blocks
An element is a pure substance consisting entirely of one type of atom. Atoms are the fundamental units of matter, indivisible by chemical means. Each element is identified by its atomic number, which represents the number of protons in its nucleus. Examples include oxygen (O), nitrogen (N), hydrogen (H), and iron (Fe). Elements are represented on the periodic table, a fundamental tool in chemistry.
Compound: Elements Bonded Together
A compound is a substance formed when two or more different elements are chemically bonded together in a fixed ratio. These bonds involve the sharing or transfer of electrons between atoms, creating a new substance with properties distinct from its constituent elements. Water (H₂O), for example, is a compound composed of two hydrogen atoms and one oxygen atom bonded together covalently. The properties of water are vastly different from the properties of hydrogen and oxygen gases. The ratio of elements in a compound is always consistent; water is always H₂O, never something else.
Mixture: A Blend of Substances
A mixture is a combination of two or more substances that are physically mixed but not chemically bonded. The substances retain their individual properties, and their proportions can vary. Unlike compounds, mixtures can be separated by physical means, such as filtration, distillation, or evaporation. Examples of mixtures include saltwater (salt and water), air, and soil. The ratio of components in a mixture is not fixed; saltwater can be made with varying amounts of salt and water.
The Composition of Air: A Detailed Examination
Now that we've established the basic classifications, let's examine the composition of air. Air is primarily a mixture of gases, but it also contains small amounts of liquid water and solid particles. The main components are:
1. Nitrogen (N₂): The Dominant Component
Nitrogen accounts for approximately 78% of the volume of dry air. It's a diatomic molecule, meaning two nitrogen atoms are bonded together covalently. While vital for plant growth and a key component of many organic molecules, nitrogen in its gaseous form (N₂) is relatively inert, meaning it doesn't readily react with other substances. This inertness is crucial for the stability of our atmosphere.
2. Oxygen (O₂): Essential for Life
Oxygen, comprising about 21% of dry air, is the second most abundant gas. It's also a diatomic molecule, with two oxygen atoms covalently bonded. Unlike nitrogen, oxygen is highly reactive, essential for respiration in most living organisms. It plays a vital role in combustion and many other chemical processes.
3. Argon (Ar): An Inert Noble Gas
Argon makes up about 0.93% of dry air. It's a noble gas, meaning it's chemically inert and doesn't readily react with other substances. Its presence in air is largely a consequence of radioactive decay processes within the Earth.
4. Other Gases: Trace Components with Significant Impacts
Besides nitrogen, oxygen, and argon, air contains trace amounts of other gases, including:
-
Carbon Dioxide (CO₂): While present in a small percentage (around 0.04%), carbon dioxide is a crucial greenhouse gas, playing a significant role in regulating Earth's temperature. Human activities have significantly increased atmospheric CO₂ levels, contributing to climate change.
-
Neon (Ne), Helium (He), Methane (CH₄), Krypton (Kr), Hydrogen (H₂), and Xenon (Xe): These are present in even smaller quantities but still contribute to the overall composition of air. Methane, like carbon dioxide, is a potent greenhouse gas, though present at lower concentrations.
-
Water Vapor (H₂O): The amount of water vapor in air varies significantly depending on location and weather conditions. It can range from near zero to several percent by volume. Water vapor plays a crucial role in the Earth's climate system and the hydrological cycle.
-
Aerosols: These are tiny solid or liquid particles suspended in the air, including dust, pollen, sea salt, and pollutants. Aerosols can have a significant impact on air quality, cloud formation, and climate.
Why Air is a Mixture, Not a Compound
The crucial point differentiating air from a compound is the variable composition and the lack of chemical bonding between its constituents. The ratio of nitrogen, oxygen, and other gases in air isn't fixed. It can vary slightly depending on altitude, location, and weather conditions. Furthermore, the gases in air are not chemically bonded to each other. They exist as individual molecules, free to move independently. If air were a compound, it would have a fixed chemical formula and consistent properties regardless of its location or conditions.
The Importance of Air Composition and Its Significance
Understanding the composition of air is vital for numerous reasons:
-
Environmental Monitoring: Tracking changes in the composition of air, particularly the levels of greenhouse gases and pollutants, is crucial for monitoring environmental health and addressing issues like climate change and air pollution.
-
Medical Applications: The precise composition of air is crucial for medical applications, such as providing respiratory support to patients and maintaining sterile environments in hospitals.
-
Industrial Processes: Many industrial processes rely on specific air compositions, for example, in combustion, welding, and the production of certain materials.
-
Climate Science: Studying the changes in air composition helps climate scientists model future climate scenarios and understand the impacts of human activity on the planet.
Conclusion: Air – A Dynamic and Essential Mixture
In conclusion, air is unequivocally a mixture, not an element or a compound. Its dynamic composition, a blend of various gases, water vapor, and aerosols, is essential for life on Earth. Understanding the intricate details of this mixture, including its variability and the impact of its constituents, is paramount for addressing various scientific, environmental, and societal challenges. Continuous monitoring and research on air composition are vital for ensuring a healthy planet and a sustainable future. The seemingly simple question of whether air is an element, compound, or mixture leads to a deep and fascinating exploration of chemistry, environmental science, and the very air we breathe.
Latest Posts
Latest Posts
-
7 5a 4 1 14 8a
Mar 18, 2025
-
The Head Of The Femur Articulates With The
Mar 18, 2025
-
What Is The Least Common Multiple Of 8 And 2
Mar 18, 2025
-
What Determines The Shape Of A Protein
Mar 18, 2025
-
How Much Is 70 Degrees Fahrenheit In Celsius
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Air An Element Or Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
