How Many Sigma And Pi Bonds In A Triple Bond
listenit
Mar 15, 2025 · 5 min read
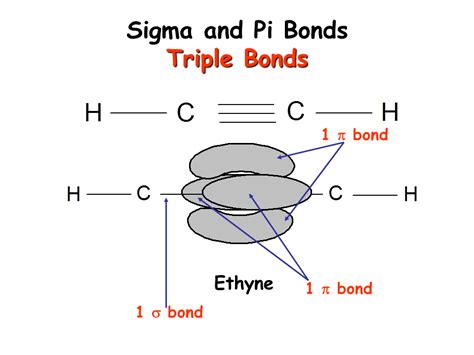
Table of Contents
How Many Sigma and Pi Bonds in a Triple Bond? A Deep Dive into Chemical Bonding
Understanding chemical bonding is fundamental to grasping the behavior of molecules. Among the various types of bonds, triple bonds represent a strong and significant form of covalent bonding, crucial in many organic and inorganic compounds. This article delves deep into the structure of triple bonds, focusing specifically on the number of sigma (σ) and pi (π) bonds present within them. We'll explore the concepts behind sigma and pi bonds, analyze examples, and consider the implications of triple bonding in different molecular contexts.
Understanding Sigma (σ) and Pi (π) Bonds
Before we delve into triple bonds, let's establish a clear understanding of sigma and pi bonds. These are types of covalent bonds formed by the overlap of atomic orbitals.
Sigma Bonds (σ Bonds): The Foundation of Covalent Bonds
A sigma bond is formed by the head-on overlap of atomic orbitals. This means the electron density is concentrated along the internuclear axis, the imaginary line connecting the two bonded atoms. Sigma bonds are the strongest type of covalent bond and are always formed first when atoms bond covalently. They are fundamental to the formation of single bonds and are also present in multiple bonds. Think of them as the backbone of the bond.
Pi Bonds (π Bonds): Adding Strength and Stability
A pi bond is formed by the sideways overlap of atomic orbitals. Unlike sigma bonds, the electron density in a pi bond is concentrated above and below the internuclear axis. Pi bonds are weaker than sigma bonds because the sideways overlap is less effective than the head-on overlap. Pi bonds are only formed after a sigma bond is already established. They add extra strength and stability to a molecule but cannot exist independently.
The Structure of a Triple Bond: Sigma and Pi Contributions
Now, let's focus on the core topic: triple bonds. A triple bond is a type of covalent bond where three pairs of electrons are shared between two atoms. This strong bond significantly impacts the molecule's properties, including bond length, bond strength, and reactivity.
The crucial point to understand is that a triple bond always consists of one sigma (σ) bond and two pi (π) bonds. The sigma bond is formed initially through head-on overlap, providing the fundamental connection between the two atoms. Then, two pi bonds are formed through sideways overlap of p-orbitals, adding extra electron density and further strengthening the bond.
In summary: Triple bond = 1 σ bond + 2 π bonds
Visualizing Triple Bonds: Examples with Molecular Orbitals
Let's visualize this with some common examples.
Acetylene (Ethyne): A Classic Example
Acetylene (C₂H₂), also known as ethyne, is a simple hydrocarbon featuring a triple bond between the two carbon atoms. Each carbon atom has two p-orbitals available for bonding. One p-orbital from each carbon undergoes head-on overlap, creating a sigma bond. The remaining two p-orbitals from each carbon then undergo sideways overlap, creating two pi bonds. This results in a stable triple bond connecting the two carbon atoms. The hydrogen atoms bond to each carbon through sigma bonds.
Nitrogen Gas (N₂): A Highly Stable Molecule
Nitrogen gas (N₂) exists as a diatomic molecule with a triple bond between the two nitrogen atoms. This exceptionally strong triple bond explains nitrogen's relative inertness and its abundance in the Earth's atmosphere. The bond formation follows the same principle as in acetylene, with one sigma bond and two pi bonds contributing to the overall triple bond.
Cyanide Ion (CN⁻): A Linear Anion
The cyanide ion (CN⁻) is a linear anion with a triple bond between the carbon and nitrogen atoms. It showcases the versatility of triple bonds in various chemical species. The bonding mechanism mirrors that of the previous examples: one sigma bond and two pi bonds provide the triple bond.
The Implications of Triple Bonds: Properties and Reactivity
The presence of a triple bond significantly influences the properties and reactivity of molecules.
Bond Length: Shorter and Stronger
Triple bonds are shorter than double and single bonds due to the increased electron density and the stronger attractive forces between the bonded atoms.
Bond Strength: High Energy
Triple bonds are the strongest type of covalent bond. This high bond strength translates to a higher bond dissociation energy, meaning it requires more energy to break the bond.
Reactivity: Influence on Chemical Reactions
The presence of a triple bond strongly influences a molecule's reactivity. The electron density in the pi bonds is more accessible for reactions than the electron density in the sigma bond, making compounds with triple bonds susceptible to electrophilic addition reactions.
Linear Geometry: Spatial Arrangement
Molecules with triple bonds generally exhibit linear geometry around the atoms involved in the triple bond. This is due to the linear arrangement of atomic orbitals involved in sigma and pi bond formation.
Beyond Simple Triple Bonds: Complex Scenarios
While the basic principle of one sigma and two pi bonds remains consistent, the complexity can increase in larger and more complex molecules.
Conjugated Systems: Delocalized Pi Bonds
In conjugated systems, where alternating single and multiple bonds are present, the pi electrons are delocalized over several atoms. This delocalization affects the bond order and properties of the entire system.
Triple Bonds in Rings: Cyclic Structures
Triple bonds can also be found within cyclic structures, introducing additional geometric constraints and influencing the molecule's overall stability and reactivity.
Conclusion: The Significance of Understanding Triple Bonds
Understanding the composition of a triple bond—one sigma and two pi bonds—is crucial for comprehending molecular structure, properties, and reactivity. The strong, short nature of triple bonds leads to unique chemical behaviors. This knowledge forms the bedrock for advanced studies in organic and inorganic chemistry, including the design and synthesis of novel molecules with specific functionalities. By understanding the fundamental aspects of sigma and pi bonding, we can better predict and interpret the behavior of a vast array of chemical compounds. From the simple acetylene molecule to the complex structures found in advanced materials, the concept of the triple bond plays a significant role in shaping the world around us. Further exploration of this topic can lead to a deeper appreciation for the intricate world of molecular bonding and its impact on various scientific disciplines.
Latest Posts
Latest Posts
-
Give The Formula For Plumbous Nitrate
Mar 15, 2025
-
1 And 2 3 As A Decimal
Mar 15, 2025
-
Proteins Are Made Of Monomers Called
Mar 15, 2025
-
X 2 4 X 2 Graph
Mar 15, 2025
-
What Is The Difference Between Ion And Atom
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Sigma And Pi Bonds In A Triple Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
