What Is The Electron Configuration Of Arsenic
listenit
Mar 24, 2025 · 6 min read
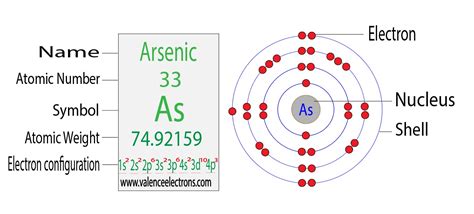
Table of Contents
What is the Electron Configuration of Arsenic? A Deep Dive into Atomic Structure
Arsenic, a metalloid element with fascinating properties and a rich history, holds a unique place on the periodic table. Understanding its electron configuration is key to unlocking its chemical behavior and various applications. This comprehensive article delves into the electron configuration of arsenic, exploring its significance and implications. We'll explore the underlying principles of electron configuration, examine arsenic's position within the periodic table, and discuss the implications of its electron arrangement for its chemical properties and reactivity.
Understanding Electron Configuration
Before diving into arsenic's specific electron configuration, let's establish a firm understanding of the fundamental concept. Electron configuration describes the arrangement of electrons within the electron shells and subshells of an atom. This arrangement dictates how an atom interacts with other atoms, forming chemical bonds and influencing its overall properties.
The Aufbau Principle and Hund's Rule
Two key principles govern electron configuration: the Aufbau principle and Hund's rule. The Aufbau principle states that electrons fill the lowest energy levels first, progressing to higher energy levels as they are filled. This means electrons will occupy the 1s orbital before the 2s, the 2s before the 2p, and so on.
Hund's rule, on the other hand, dictates that electrons will individually occupy each orbital within a subshell before pairing up in any one orbital. This minimizes electron-electron repulsion and leads to a more stable configuration.
Electron Shells and Subshells
Electrons reside in shells, denoted by the principal quantum number (n), which can take integer values (1, 2, 3, etc.). Each shell consists of subshells, designated by the letters s, p, d, and f. These subshells have different shapes and can hold varying numbers of electrons:
- s subshell: spherical shape, holds a maximum of 2 electrons.
- p subshell: dumbbell shape, holds a maximum of 6 electrons (3 orbitals).
- d subshell: more complex shape, holds a maximum of 10 electrons (5 orbitals).
- f subshell: even more complex shape, holds a maximum of 14 electrons (7 orbitals).
Arsenic's Position and Properties
Arsenic (As) is located in Group 15 (or VA) and Period 4 of the periodic table. Its atomic number is 33, meaning it has 33 protons and 33 electrons in a neutral atom. This position dictates its electron configuration and its chemical behavior. Being a metalloid, arsenic exhibits properties of both metals and nonmetals. It's a semiconductor, meaning its electrical conductivity lies between that of conductors and insulators. It's also relatively toxic, with several allotropic forms exhibiting varying degrees of reactivity.
Determining Arsenic's Electron Configuration
Applying the Aufbau principle and Hund's rule, we can determine the electron configuration of arsenic:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p³
Let's break this down:
- 1s²: Two electrons fill the first energy level's s subshell.
- 2s²: Two electrons fill the second energy level's s subshell.
- 2p⁶: Six electrons fill the second energy level's p subshell (2 electrons in each of the three p orbitals).
- 3s²: Two electrons fill the third energy level's s subshell.
- 3p⁶: Six electrons fill the third energy level's p subshell.
- 4s²: Two electrons fill the fourth energy level's s subshell.
- 3d¹⁰: Ten electrons fill the third energy level's d subshell. Note that the 3d subshell fills after the 4s subshell despite being in a lower energy level. This is due to the complex interplay of energy levels.
- 4p³: Three electrons partially fill the fourth energy level's p subshell, each occupying a separate p orbital according to Hund's rule.
This configuration reflects arsenic's position in the periodic table and explains its chemical behavior. The three electrons in the 4p subshell are valence electrons, the electrons involved in chemical bonding. These valence electrons contribute to arsenic's ability to form various compounds and participate in chemical reactions.
Noble Gas Configuration
A simplified way to represent arsenic's electron configuration is using the noble gas configuration. Noble gases are characterized by their full valence shells, making them exceptionally stable. We can use the noble gas preceding arsenic, argon (Ar), to represent the filled inner shells:
[Ar] 4s² 3d¹⁰ 4p³
This notation indicates that arsenic's inner electrons are arranged like those of argon, with the remaining electrons occupying the 4s, 3d, and 4p subshells.
Implications of Arsenic's Electron Configuration
Arsenic's electron configuration has profound implications for its chemical properties and reactivity:
-
Variable Oxidation States: The presence of three valence electrons in the 4p subshell allows arsenic to exhibit various oxidation states, including -3, +3, and +5. This ability to gain or lose electrons contributes to its diverse chemistry.
-
Covalent Bonding: Arsenic's tendency to share electrons with other atoms through covalent bonds is directly related to its incomplete 4p subshell. This explains its ability to form numerous covalent compounds with other elements.
-
Semiconductor Properties: The arrangement of electrons in arsenic's outer shell contributes to its semiconductor properties. The energy gap between the valence band (filled orbitals) and the conduction band (unfilled orbitals) allows controlled electrical conductivity, making it useful in various electronic applications.
-
Toxicity: The reactivity of arsenic, linked to its electron configuration, plays a crucial role in its toxicity. Arsenic compounds can interfere with biological processes by binding to crucial enzymes and disrupting cellular function.
-
Allotropes: Arsenic exists in various allotropic forms, with differences in physical and chemical properties stemming from subtle differences in atomic arrangements.
Arsenic in Everyday Life and Applications
Despite its toxicity, arsenic finds applications in several industrial settings. Historically, arsenic compounds have been used in pesticides, but their toxicity has led to stricter regulations and the search for safer alternatives. However, arsenic continues to have niche applications in:
-
Semiconductors: Arsenic is used in certain semiconductor alloys, enhancing their electrical properties.
-
Medicine (Historically): Arsenic compounds have historically had limited use in medicine, though modern medicine largely avoids its use due to its toxicity and the availability of safer alternatives.
-
Alloys: Arsenic can be used in small quantities to modify the properties of certain metal alloys.
-
Wood Preservatives (Historically): Arsenic compounds have been used as wood preservatives, although the high toxicity has largely prompted phasing out their use.
Conclusion
The electron configuration of arsenic, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p³, dictates its chemical behavior and many of its properties. Understanding this configuration is crucial for comprehending arsenic's role in various applications, including its use in semiconductors, and its historical, albeit controversial, role in medicine and other fields. Its variable oxidation states, ability to form covalent bonds, and semiconductor properties all stem from the arrangement of its 33 electrons. While its toxicity requires cautious handling, its unique properties continue to make arsenic a significant element in specific industrial contexts. Further research continues to explore its potential benefits while mitigating its risks. This detailed exploration of arsenic's electron configuration and its implications highlights the importance of understanding atomic structure in explaining the chemical behavior and application of elements within the periodic table.
Latest Posts
Latest Posts
-
What Are The Factors For 43
Mar 26, 2025
-
How To Convert Polar Equations To Rectangular Form
Mar 26, 2025
-
What Is The Least Common Multiple Of 12 And 2
Mar 26, 2025
-
What Type Of Bond Involves The Unequal Sharing Of Electrons
Mar 26, 2025
-
Sum Of Interior Angles In A Heptagon
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Arsenic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
