What Type Of Bond Involves The Unequal Sharing Of Electrons
listenit
Mar 26, 2025 · 7 min read
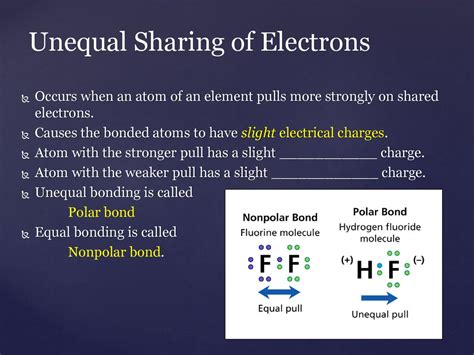
Table of Contents
What Type of Bond Involves the Unequal Sharing of Electrons?
A polar covalent bond is a type of chemical bond where a pair of electrons is unequally shared between two atoms. This unequal sharing creates a polar molecule, meaning it has a slightly positive end and a slightly negative end. This difference in electronegativity, the tendency of an atom to attract electrons towards itself, is the driving force behind the formation of polar covalent bonds. Understanding polar covalent bonds is crucial to comprehending the properties and behavior of countless molecules in chemistry and beyond.
Understanding Electronegativity and its Role in Bond Formation
Before delving deeper into polar covalent bonds, let's clarify the concept of electronegativity. Electronegativity is a fundamental property of atoms that reflects their ability to attract electrons within a chemical bond. Elements with high electronegativity strongly pull electrons towards themselves, while elements with low electronegativity hold onto electrons less tightly. The Pauling scale, a widely used system, quantifies electronegativity with fluorine (F) assigned the highest value of 4.0.
The difference in electronegativity between two atoms involved in a bond directly determines the type of bond formed. A large difference leads to an ionic bond, where electrons are essentially transferred from one atom to another, creating ions with opposite charges. A small difference results in a nonpolar covalent bond, where electrons are shared almost equally between the atoms. However, when the difference in electronegativity falls within a certain intermediate range, a polar covalent bond is formed.
The Characteristics of Polar Covalent Bonds
Polar covalent bonds exhibit several key characteristics that distinguish them from ionic and nonpolar covalent bonds:
-
Unequal Electron Sharing: The defining feature of a polar covalent bond is the unequal sharing of electrons. The atom with higher electronegativity attracts the shared electrons more strongly, resulting in a greater electron density around that atom.
-
Partial Charges: Because of the unequal electron distribution, the atom with higher electronegativity acquires a partial negative charge (δ-), while the atom with lower electronegativity develops a partial positive charge (δ+). These are not full charges like in ionic bonds, but rather represent a shift in electron density.
-
Dipole Moment: The separation of charges in a polar molecule creates a dipole moment, a vector quantity representing the magnitude and direction of the charge separation. The dipole moment is often represented by an arrow pointing from the positive to the negative end of the molecule.
-
Polarity Influences Properties: The polarity of molecules significantly influences their physical and chemical properties, including melting point, boiling point, solubility, and reactivity. Polar molecules tend to have higher boiling points and melting points than nonpolar molecules because of stronger intermolecular forces (dipole-dipole interactions and hydrogen bonding). They are also generally more soluble in polar solvents like water than in nonpolar solvents.
Examples of Polar Covalent Bonds
Many common molecules contain polar covalent bonds. Here are some examples:
-
Water (H₂O): Oxygen (O) is significantly more electronegative than hydrogen (H). Therefore, the electrons in the O-H bonds are pulled more towards the oxygen atom, making it partially negative (δ-) and the hydrogen atoms partially positive (δ+). This polarity is responsible for many of water's unique properties.
-
Hydrogen Fluoride (HF): Fluorine (F), the most electronegative element, strongly attracts the electrons in the H-F bond, leading to a highly polar molecule.
-
Ammonia (NH₃): Nitrogen (N) is more electronegative than hydrogen (H), resulting in polar N-H bonds. The overall geometry of the ammonia molecule also contributes to its significant polarity.
-
Hydrogen Chloride (HCl): Chlorine (Cl) is more electronegative than hydrogen (H), leading to a polar H-Cl bond and a polar molecule.
-
Carbon Monoxide (CO): Although carbon and oxygen are both non-metals, the electronegativity difference between them results in a polar covalent bond, with oxygen carrying a partial negative charge.
Differentiating Polar Covalent Bonds from Other Bond Types
It's important to distinguish polar covalent bonds from ionic and nonpolar covalent bonds:
Polar Covalent vs. Ionic Bonds:
The key difference lies in the degree of electron sharing. In polar covalent bonds, electrons are shared unequally, while in ionic bonds, electrons are essentially transferred from one atom to another. The electronegativity difference is much larger in ionic bonds than in polar covalent bonds. Ionic bonds typically form between metals and nonmetals, while polar covalent bonds usually form between nonmetals with different electronegativities.
Polar Covalent vs. Nonpolar Covalent Bonds:
In nonpolar covalent bonds, electrons are shared almost equally between atoms. This occurs when the atoms have similar electronegativities, often when they are the same element (e.g., H₂ or O₂). Polar covalent bonds, on the other hand, involve unequal electron sharing due to a significant difference in electronegativity between the atoms involved.
The Impact of Polarity on Molecular Properties
The polarity of a molecule significantly influences various properties:
Solubility:
Polar molecules tend to dissolve in polar solvents (like water) because of the attractive forces between the partial charges of the solute and solvent molecules. This is often described as "like dissolves like". Nonpolar molecules, however, are more soluble in nonpolar solvents.
Boiling and Melting Points:
Polar molecules generally have higher boiling and melting points than nonpolar molecules of similar size. This is because the stronger dipole-dipole interactions and hydrogen bonds (a special type of dipole-dipole interaction) require more energy to overcome.
Surface Tension and Viscosity:
Polarity influences the surface tension and viscosity of liquids. Polar molecules, due to stronger intermolecular forces, tend to exhibit higher surface tension and viscosity.
Reactivity:
The polarity of molecules affects their chemical reactivity. Polar molecules are often more reactive than nonpolar molecules because the partial charges can participate in chemical reactions more readily.
Hydrogen Bonding: A Special Case of Polar Covalent Bonds
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a different molecule. This is a crucial intermolecular force that significantly impacts the properties of many molecules, including water, proteins, and DNA. The unusually high boiling point of water is a direct consequence of its strong hydrogen bonding network.
Beyond the Basics: Factors Affecting Polarity
While the electronegativity difference is the primary determinant of bond polarity, other factors also influence the overall polarity of a molecule:
-
Molecular Geometry: Even if a molecule contains polar bonds, the overall molecular polarity depends on the arrangement of these bonds in three-dimensional space. Symmetrical molecules can have zero net dipole moment despite containing polar bonds (e.g., carbon dioxide, CO₂).
-
Bond Length: The length of a bond can subtly influence the degree of polarity. Longer bonds might exhibit slightly less pronounced polarity due to increased electron delocalization.
-
Resonance: In molecules with resonance structures, the distribution of electrons is delocalized, which can affect the overall polarity of the molecule.
Applications of Polar Covalent Bonds
Understanding polar covalent bonds is critical in various scientific and technological applications:
-
Drug Discovery: The polarity of drug molecules plays a significant role in their absorption, distribution, metabolism, and excretion in the body.
-
Materials Science: The properties of many materials, including polymers and semiconductors, are directly linked to the polarity of the constituent molecules.
-
Environmental Science: The polarity of molecules influences their environmental behavior, including their transport in soil and water and their interactions with living organisms.
-
Food Science: The polarity of food molecules impacts their texture, flavor, and preservation.
-
Cosmetics and Personal Care: Polarity plays a role in the formulation and effectiveness of many cosmetic and personal care products.
Conclusion: The Significance of Polar Covalent Bonds
Polar covalent bonds are a fundamental aspect of chemistry, impacting the structure, properties, and reactivity of countless molecules. Understanding the concept of electronegativity and its role in determining the type of bond formed is essential for grasping the behavior of substances at both the molecular and macroscopic levels. The unequal sharing of electrons in polar covalent bonds leads to a diverse range of physical and chemical properties, with far-reaching implications in various fields of science and technology. The interplay of electronegativity, molecular geometry, and other factors contributes to the complex and fascinating world of molecular interactions. Further exploration into this topic unveils the intricate details that govern the behavior of matter and the universe around us.
Latest Posts
Latest Posts
-
6 Of 15 Is What Percent
Mar 26, 2025
-
Systems Of Equations With Three Variables
Mar 26, 2025
-
Greatest Common Factor Of 28 And 42
Mar 26, 2025
-
Does Nitrogen Follow The Octet Rule
Mar 26, 2025
-
Is 37 A Prime Number Or A Composite Number
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bond Involves The Unequal Sharing Of Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
