Does Nitrogen Follow The Octet Rule
listenit
Mar 26, 2025 · 5 min read
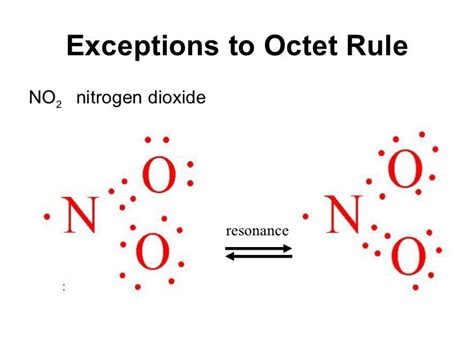
Table of Contents
Does Nitrogen Follow the Octet Rule? A Deep Dive into Nitrogen's Bonding Behavior
Nitrogen, a ubiquitous element crucial for life as we know it, often sparks discussions regarding its adherence to the octet rule. This rule, a cornerstone of basic chemistry, dictates that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons, mimicking the noble gas configuration. While seemingly straightforward, nitrogen's behavior presents intriguing nuances that warrant a closer examination. This article will explore the complexities of nitrogen's bonding and delve into the exceptions to the octet rule that it exhibits.
Understanding the Octet Rule and its Limitations
The octet rule stems from the stability associated with filled electron shells. Atoms strive for this stability, often achieving it through chemical bonding. This is particularly evident in the second period elements, where obtaining eight valence electrons fills their outermost s and p orbitals. However, the octet rule is not a rigid law; rather, it's a useful guideline with several important exceptions. Elements in the third period and beyond, with access to d orbitals, can frequently accommodate more than eight electrons in their valence shell, a phenomenon known as expanded octet. Conversely, some elements, notably those in the second period like nitrogen, can exhibit incomplete octets, possessing fewer than eight valence electrons in certain compounds.
Nitrogen's Electron Configuration and Bonding Capabilities
Nitrogen, with an atomic number of 7, possesses an electron configuration of 1s²2s²2p³. This implies five valence electrons, three of which are unpaired in the 2p orbitals. This unpaired electron configuration drives nitrogen's tendency to form covalent bonds, sharing electrons to achieve a more stable electronic arrangement. Nitrogen's strong electronegativity further contributes to its diverse bonding patterns.
Common Bonding Scenarios for Nitrogen: Adhering to the Octet Rule
In many of its compounds, nitrogen successfully achieves an octet. Let's examine some prime examples:
-
Ammonia (NH₃): In ammonia, nitrogen forms three single covalent bonds with three hydrogen atoms. Each hydrogen atom contributes one electron, and nitrogen shares three electrons, resulting in a complete octet for nitrogen (two electrons from the 2s orbital and six from the three covalent bonds). The nitrogen atom also possesses a lone pair of electrons.
-
Ammonium Ion (NH₄⁺): The ammonium ion extends this bonding pattern. Nitrogen forms four covalent bonds with four hydrogen atoms, achieving a complete octet. The positive charge signifies the loss of one electron, leaving a full shell of eight.
-
Nitriles (R-CN): In nitriles, nitrogen forms a triple bond with a carbon atom, using three of its five valence electrons. The remaining two electrons form a lone pair, again fulfilling the octet rule.
These examples highlight nitrogen's frequent compliance with the octet rule, underscoring its ability to attain stability through covalent bonding. However, the story doesn't end here.
Exceptions to the Octet Rule: Where Nitrogen Deviates
While nitrogen often achieves an octet, certain situations demonstrate its capacity to deviate from this guideline:
-
Nitric Oxide (NO): This molecule contains an unpaired electron on the nitrogen atom, resulting in a total of seven valence electrons around nitrogen. This constitutes an incomplete octet. The unpaired electron contributes to nitric oxide's paramagnetism.
-
Nitrogen Monoxide (N₂O): Similar to nitric oxide, the central nitrogen atom in N₂O doesn't quite reach an octet. The resonance structures depict a variation in bond order, influencing the electron distribution and leading to an incomplete octet for the nitrogen.
-
Nitrogen Dioxide (NO₂): Nitrogen dioxide exhibits a similar scenario. The presence of an unpaired electron around the nitrogen atom leaves it with an incomplete octet. This contributes to its radical nature and reactivity.
These exceptions underscore that while the octet rule serves as a valuable tool, it's not a universally applicable rule for all nitrogen compounds. The electronic structure of the molecule and the competing factors governing bond formation influence whether nitrogen adheres to the octet rule.
Factors Influencing Nitrogen's Octet Behavior
Several key factors influence whether nitrogen adheres to the octet rule in a particular molecule:
-
Electronegativity: Nitrogen's relatively high electronegativity plays a significant role. It strongly attracts electrons, making it likely to form covalent bonds that contribute to achieving an octet.
-
Bonding Capacity: Nitrogen's ability to form three covalent bonds and possess one lone pair allows it flexibility in achieving an octet or deviating from it.
-
Resonance: In some molecules, resonance structures contribute to the electron delocalization, affecting the electron count around the nitrogen atom and influencing its proximity to fulfilling an octet.
-
Steric Factors: The spatial arrangement of atoms in a molecule can also influence bond formation and electron distribution around nitrogen.
The Importance of Understanding Nitrogen's Bonding
Understanding nitrogen's bonding behavior, including its adherence to and deviations from the octet rule, is crucial for several reasons:
-
Predicting Molecular Geometry: Knowledge of the electron distribution around nitrogen allows us to predict the molecular geometry using VSEPR theory.
-
Explaining Reactivity: Understanding whether nitrogen follows the octet rule helps predict its reactivity in different chemical contexts. Molecules with incomplete octets are often more reactive due to the presence of unpaired electrons.
-
Designing New Compounds: Knowledge of nitrogen's bonding is essential in the design and synthesis of new molecules with specific properties.
-
Understanding Biological Processes: Nitrogen's role in biological molecules, like amino acids and nucleic acids, is directly linked to its bonding behavior. Understanding its bonding patterns is crucial to comprehending life processes.
Conclusion: A Flexible Rule, Not a Rigid Law
The question of whether nitrogen follows the octet rule is not a simple yes or no answer. While nitrogen frequently achieves a stable octet through covalent bonding, it also exhibits notable exceptions. The decision of whether it adheres to the rule depends on various factors, including electronegativity, bonding capacity, resonance, and steric factors. The octet rule serves as a useful guideline, but its limitations should be acknowledged, particularly in understanding the diverse bonding patterns of elements like nitrogen. A deep understanding of these intricacies allows for accurate predictions of molecular properties and reactivity, paving the way for advancements in various scientific fields. Therefore, appreciating the complexities of nitrogen's bonding behavior is not merely an academic exercise; it's a crucial foundation for progress in chemistry and related disciplines.
Latest Posts
Latest Posts
-
Examples Of Combustion In Everyday Life
Mar 29, 2025
-
Graph Of X 2y Y 2
Mar 29, 2025
-
Why Is Density A Derived Unit
Mar 29, 2025
-
Is Density A Physical Or Chemical Change
Mar 29, 2025
-
What Plane Divides The Body Into Anterior And Posterior Parts
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Does Nitrogen Follow The Octet Rule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
