Why Is Density A Derived Unit
listenit
Mar 29, 2025 · 5 min read
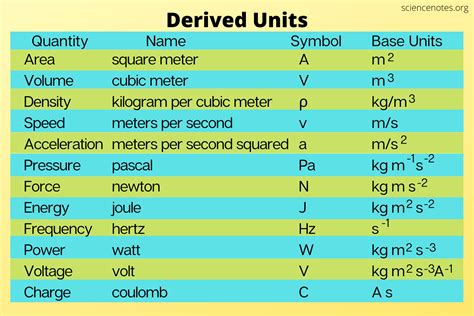
Table of Contents
Why is Density a Derived Unit? Understanding Fundamental and Derived Quantities in Physics
Density, a fundamental concept in physics and chemistry, is often described as a derived unit. But what exactly does that mean, and why isn't it considered a fundamental unit like mass or length? This article will delve deep into the nature of density, exploring its relationship to fundamental units and explaining why it's classified as derived. We'll also explore the practical implications of understanding density as a derived quantity and its importance across various scientific disciplines.
Understanding Fundamental and Derived Units
Before we dive into the specifics of density, let's establish a clear understanding of fundamental and derived units. In the International System of Units (SI), the seven fundamental units form the basis of all other measurements. These are:
- Meter (m): Unit of length
- Kilogram (kg): Unit of mass
- Second (s): Unit of time
- Ampere (A): Unit of electric current
- Kelvin (K): Unit of thermodynamic temperature
- Mole (mol): Unit of amount of substance
- Candela (cd): Unit of luminous intensity
These fundamental units are independent of each other; they aren't defined in terms of any other unit. They are the building blocks of the entire system.
Derived units, on the other hand, are constructed from combinations of fundamental units. They represent physical quantities that are not considered fundamental but are essential for describing various physical phenomena. Examples include:
- Velocity (m/s): Derived from length (meter) and time (second).
- Force (kg⋅m/s² or Newton): Derived from mass, length, and time.
- Energy (kg⋅m²/s² or Joule): Derived from mass, length, and time.
Density: A Deep Dive into its Definition and Calculation
Density, denoted by the Greek letter ρ (rho), is defined as the mass per unit volume of a substance. This seemingly simple definition holds the key to understanding why density is a derived unit. Let's break down the formula:
ρ = m/V
Where:
- ρ represents density
- m represents mass (measured in kilograms, kg - a fundamental unit)
- V represents volume (measured in cubic meters, m³ - a derived unit itself, based on the fundamental unit of length)
As you can see, the calculation of density directly involves both mass and volume. Since mass is a fundamental unit (kg) and volume (m³) is a derived unit (length x length x length), density inherently inherits its derived nature. It's a combination of fundamental and derived units, making it, in essence, a derived unit itself.
Why Volume is a Derived Unit
It's crucial to understand why volume is a derived unit. Volume represents the three-dimensional space occupied by a substance. It's calculated by multiplying length, width, and height. Since length is a fundamental unit (meter), volume is a combination of three fundamental units:
Volume = Length x Width x Height = m x m x m = m³
Therefore, volume (m³) is intrinsically derived from the fundamental unit of length (meter).
The Units of Density: A Reflection of its Derived Nature
The unit of density is typically expressed as kilograms per cubic meter (kg/m³) in the SI system. This unit directly reflects the formula: mass (kg) divided by volume (m³). Other units for density, like grams per cubic centimeter (g/cm³), are also derived units, merely using different combinations of fundamental units. The essential point remains: the unit of density isn't a single, independent fundamental unit but a combination derived from others.
Practical Implications of Understanding Density as a Derived Unit
The understanding of density as a derived unit is crucial in several scientific and engineering applications:
-
Material Science: Density is a fundamental property of materials, helping differentiate between different substances. Knowing it’s a derived unit allows for precise calculations involving mass and volume, critical for material selection and design.
-
Fluid Mechanics: Density plays a crucial role in determining buoyancy, fluid flow, and pressure. Understanding its derived nature aids in accurate modeling and prediction of fluid behavior.
-
Chemical Engineering: Density is used extensively in process calculations, particularly in determining the concentration of solutions, mixing ratios, and flow rates. Its derived nature ensures consistency and accuracy in such calculations.
Density and Dimensional Analysis
Dimensional analysis is a powerful tool in physics that utilizes the dimensions (fundamental units) of physical quantities to check the consistency of equations and derive relationships between different quantities. Density, being a derived unit, perfectly demonstrates the application of dimensional analysis. The formula ρ = m/V can be expressed dimensionally as:
[ρ] = [m]/[V] = M/L³
Where:
- M represents the dimension of mass.
- L represents the dimension of length.
This dimensional analysis confirms that density has dimensions of mass per unit volume, reinforcing its derived nature.
Comparing Density to Other Derived Quantities
Let's compare density to other commonly used derived quantities to further emphasize its derived nature. Consider velocity (v = d/t) with units of meters per second (m/s). It's derived from length (m) and time (s), two fundamental units. Similarly, acceleration (a = Δv/t) combines length and time to produce units of meters per second squared (m/s²). All these quantities, including density, are constructed from fundamental units, highlighting their status as derived quantities.
Density in Different Units and Conversion Factors
While kg/m³ is the standard SI unit for density, many other units exist depending on the context. For instance, g/cm³ is frequently used in chemistry, and lb/ft³ is common in engineering applications. The ability to convert between these different units highlights the derived nature of density. The conversion factors always involve the fundamental units of mass and length.
Conclusion: The Importance of Understanding Derived Units
The fact that density is a derived unit isn't merely a matter of classification; it's a fundamental aspect of its definition and practical application. Its derived status reflects its dependency on fundamental units of mass and length, emphasizing its role in a broader system of measurement. Understanding density's derived nature is not just a theoretical exercise; it’s crucial for performing accurate calculations, building reliable models, and making sound decisions across numerous scientific and engineering fields. By grasping the relationship between fundamental and derived units, we gain a deeper appreciation for the coherence and interconnectedness of physical quantities and the system of measurement that describes them. The seemingly simple concept of density serves as a powerful illustration of this fundamental principle.
Latest Posts
Latest Posts
-
Which Type Of Chemical Bond Involves The Exchange Of Electrons
Mar 31, 2025
-
What Is The Charge For Chlorine
Mar 31, 2025
-
Finding Mole Ratio Practice Questions 1 Answer Key
Mar 31, 2025
-
How Many Atoms Are In Nitrogen
Mar 31, 2025
-
How Many Valence Electrons Does Gallium Have
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Why Is Density A Derived Unit . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
