What Is The Charge For Chlorine
listenit
Mar 31, 2025 · 5 min read
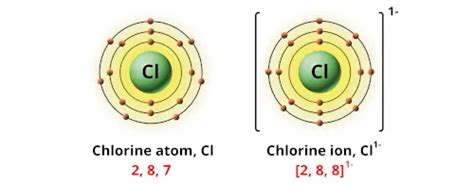
Table of Contents
What is the Charge for Chlorine? Understanding Chlorine's Oxidation States
Chlorine, a ubiquitous element crucial in various industrial and domestic applications, doesn't possess a single fixed charge. Instead, its charge, or more accurately, its oxidation state, varies depending on the chemical context. Understanding chlorine's variable oxidation states is vital for comprehending its diverse reactivity and applications. This comprehensive guide explores the different oxidation states of chlorine, their implications, and the factors influencing them.
Chlorine's Versatile Nature: A Multifaceted Element
Chlorine (Cl), a halogen residing in Group 17 of the periodic table, boasts seven electrons in its outermost shell. This electronic configuration dictates its strong tendency to gain one electron to achieve a stable octet, resembling the noble gas argon. This electron gain results in the formation of the chloride ion (Cl⁻), exhibiting an oxidation state of -1. This is the most common oxidation state for chlorine and is observed in numerous compounds. However, chlorine's reactivity extends beyond this simple electron gain. Its ability to share electrons or even lose electrons under specific conditions gives rise to a variety of oxidation states.
The Common Oxidation State: -1 (Chloride Ion)
The -1 oxidation state is undoubtedly the most prevalent for chlorine. In this state, chlorine exists as the chloride ion (Cl⁻), a negatively charged species. This ion forms readily when chlorine reacts with metals or less electronegative non-metals. Examples of compounds where chlorine exhibits a -1 oxidation state include:
- Sodium chloride (NaCl): Common table salt, a crucial electrolyte in biological systems.
- Hydrogen chloride (HCl): A strong acid, vital in industrial processes and laboratory settings. In aqueous solution, it forms hydrochloric acid.
- Calcium chloride (CaCl₂): Used as a de-icer and desiccant.
- Numerous organic chlorides: These are extensively used in various organic synthesis reactions and as solvents.
The stability of the chloride ion is the cornerstone of chlorine's widespread presence in various compounds. The strong electrostatic attraction between the positively charged metal ions and the negatively charged chloride ions leads to the formation of stable ionic compounds.
Beyond -1: Exploring Other Oxidation States of Chlorine
While -1 is the most familiar oxidation state, chlorine's versatility allows it to display a range of other oxidation states, including positive ones. These unusual oxidation states often arise in compounds with highly electronegative elements like oxygen and fluorine.
+1 Oxidation State: Hypochlorite Ion (OCl⁻)
In hypochlorite compounds, chlorine exhibits a +1 oxidation state. The hypochlorite ion (OCl⁻) is the active ingredient in many household bleaches. Here, chlorine shares electrons with oxygen, but the electronegativity difference dictates the positive charge on chlorine. The hypochlorite ion is a strong oxidizing agent, responsible for the bleaching action.
+3 Oxidation State: Chlorite Ion (ClO₂⁻)
The +3 oxidation state is found in chlorite compounds, which contain the chlorite ion (ClO₂⁻). Chlorite salts are less common than hypochlorites but still find applications as disinfectants and bleaching agents. The chlorite ion is a stronger oxidizing agent than hypochlorite.
+5 Oxidation State: Chlorate Ion (ClO₃⁻)
Chlorate compounds contain the chlorate ion (ClO₃⁻), where chlorine is in the +5 oxidation state. Chlorates are strong oxidizing agents and are used in various applications, including the production of matches and fireworks. However, caution is warranted due to their explosive potential.
+7 Oxidation State: Perchlorate Ion (ClO₄⁻)
The highest common oxidation state for chlorine is +7, found in perchlorate compounds. The perchlorate ion (ClO₄⁻) is a powerful oxidizing agent, used in various applications including rocket propellants and explosives. Perchlorates are also environmental concerns due to their persistence and potential toxicity.
Factors Influencing Chlorine's Oxidation State
Several factors determine the oxidation state chlorine adopts in a particular compound:
-
Electronegativity: Chlorine's electronegativity dictates its ability to attract electrons. When bonded to more electronegative atoms like oxygen or fluorine, it tends to exhibit positive oxidation states. Conversely, when bonded to less electronegative atoms, it favors negative oxidation states.
-
Bonding Partners: The nature of the atoms chlorine bonds with heavily influences its oxidation state. Highly electronegative atoms like oxygen and fluorine can force chlorine into positive oxidation states. Conversely, metals and less electronegative non-metals readily allow chlorine to achieve the -1 oxidation state.
-
Chemical Environment: The reaction conditions, such as pH and presence of other reactants, can also impact the oxidation state. For instance, chlorine's oxidation state can change during redox reactions.
-
Formal Charge Considerations: Although oxidation state is a useful concept, it is crucial to remember that it's a formal assignment based on electronegativity differences. The actual charge distribution in a molecule can deviate from the formal oxidation states due to resonance and other factors.
The Significance of Understanding Chlorine's Oxidation States
Understanding chlorine's varying oxidation states is pivotal for several reasons:
-
Predicting Reactivity: The oxidation state provides insights into a compound's reactivity. Higher positive oxidation states generally indicate stronger oxidizing capabilities.
-
Synthesis and Applications: Knowing the desired oxidation state helps in designing chemical reactions to synthesize specific chlorine-containing compounds. The applications of these compounds vary greatly depending on chlorine's oxidation state.
-
Environmental Concerns: Some chlorine-containing compounds, particularly those with higher oxidation states, can have environmental impacts. Understanding their reactivity and stability is crucial for managing environmental risks.
-
Biological Significance: Chlorine and its compounds play various roles in biological systems. Understanding chlorine's oxidation states helps in deciphering its role in biological processes.
Conclusion: A Comprehensive Overview
Chlorine's charge is not fixed but varies across a range of oxidation states, primarily influenced by its bonding partners and the chemical environment. While the -1 oxidation state is the most common, featuring in numerous vital compounds, chlorine's ability to exist in positive oxidation states highlights its remarkable versatility. Comprehending these different oxidation states is fundamental to understanding chlorine's reactivity, its diverse applications, and its significant role in both industrial processes and natural environments. Further research continues to unravel the intricacies of chlorine chemistry and its implications across various scientific fields.
Latest Posts
Latest Posts
-
Greatest Common Factor Of 24 And 42
Apr 02, 2025
-
What 3 Particles Make Up An Atom
Apr 02, 2025
-
Blood Is What Type Of Mixture
Apr 02, 2025
-
What Is The Empirical Formula Of Ibuprofen
Apr 02, 2025
-
What Is One Sixth As A Decimal
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge For Chlorine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
