How Many Valence Electrons Does Gallium Have
listenit
Mar 31, 2025 · 5 min read
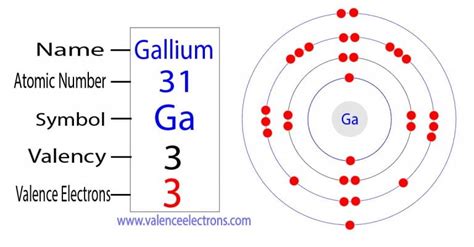
Table of Contents
How Many Valence Electrons Does Gallium Have? A Deep Dive into Gallium's Electronic Structure
Gallium, a fascinating element with a surprising number of applications, often sparks curiosity among chemistry enthusiasts. One of the most fundamental questions surrounding this metalloid revolves around its valence electrons – the electrons involved in chemical bonding and determining its reactivity. This comprehensive article will delve into the answer to the question: How many valence electrons does gallium have? We'll explore the electronic configuration, its implications for gallium's chemical behavior, and its significance in various fields.
Understanding Valence Electrons
Before we focus specifically on gallium, let's establish a clear understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of compounds it can form. The number of valence electrons directly influences an element's position within the periodic table and its properties.
Atoms strive for stability, often achieving this by having a full outermost electron shell. This is the basis of the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve eight electrons in their valence shell. However, there are exceptions, especially for elements beyond the second period.
Determining Gallium's Valence Electrons: Electronic Configuration
To determine the number of valence electrons in gallium (Ga), we need to examine its electronic configuration. Gallium has an atomic number of 31, meaning it has 31 protons and 31 electrons in a neutral atom. The electronic configuration of gallium is: 1s²2s²2p⁶3s²3p⁶3d¹⁰4s²4p¹.
This configuration reveals the distribution of electrons across various energy levels and subshells. The valence electrons are those residing in the highest energy level, which, in gallium's case, is the fourth energy level (n=4). In this level, we find two electrons in the 4s subshell and one electron in the 4p subshell.
Therefore, gallium has a total of three valence electrons (4s²4p¹).
Implications of Gallium's Three Valence Electrons
The presence of three valence electrons significantly influences gallium's chemical and physical properties. This relatively small number of valence electrons leads to several key characteristics:
1. Variable Oxidation States:
Unlike elements with a full or nearly full valence shell, gallium exhibits variable oxidation states. Its most common oxidation state is +3, reflecting the loss of its three valence electrons. However, under specific conditions, gallium can also exhibit a +1 oxidation state, losing only the 4p electron. This ability to exist in different oxidation states makes gallium a versatile element in various chemical reactions.
2. Metallic Character:
Gallium is a post-transition metal, exhibiting metallic characteristics such as good electrical and thermal conductivity. The relatively weak hold on its three valence electrons contributes to the metal's ability to conduct electricity. These electrons can move freely throughout the metallic lattice, creating a sea of delocalized electrons responsible for conductivity.
3. Formation of Compounds:
Gallium's three valence electrons allow it to form a variety of compounds. It readily participates in covalent and ionic bonding, forming compounds with various elements. For example, gallium readily reacts with halogens (such as chlorine and bromine) to form gallium halides (like GaCl₃ and GaBr₃). It also forms compounds with oxygen, sulfur, and other elements.
4. Semiconductor Properties:
Gallium's electronic structure and its ability to form compounds contribute to its semiconductor properties. Gallium arsenide (GaAs), a compound formed from gallium and arsenic, is a prominent III-V semiconductor material widely used in electronic devices, such as solar cells and LEDs. The unique electronic band structure of GaAs, influenced by the valence electron configuration of gallium, enables its semiconductor behavior.
Gallium's Applications: A Testament to its Electronic Structure
Gallium's unique properties, directly linked to its three valence electrons, have led to a broad range of applications across diverse fields. Some notable applications include:
1. Electronics and Semiconductors:
As mentioned earlier, gallium arsenide (GaAs) and other gallium-containing compounds are crucial components in semiconductor devices. These materials exhibit superior performance compared to silicon in certain applications, such as high-frequency circuits and optoelectronic devices.
2. LED Lighting:
Gallium nitride (GaN) is a key material in high-efficiency, energy-saving LED lighting. The electronic structure of gallium, specifically its three valence electrons, contributes to the unique optical properties of GaN, allowing it to emit light in the blue and ultraviolet regions of the electromagnetic spectrum.
3. Solar Cells:
Gallium arsenide solar cells are known for their high efficiency in converting sunlight into electricity. These cells outperform silicon-based solar cells in certain conditions, especially under low-light conditions and at higher temperatures.
4. Medicine:
Gallium-67, a radioactive isotope of gallium, finds application in nuclear medicine. It is used as a radiotracer to help visualize tumors and other abnormalities in the body. Its ability to accumulate in certain tissues is related to its chemical properties, which are, in turn, determined by its electronic structure.
5. Metallurgy:
Gallium's low melting point and its ability to wet other metals make it useful in various metallurgical applications. It is used in low-melting alloys, which find application in temperature sensors and other specialized applications.
Conclusion: The Significance of Gallium's Three Valence Electrons
In conclusion, gallium possesses three valence electrons, a characteristic directly responsible for its diverse chemical and physical properties. These three valence electrons enable gallium to exhibit variable oxidation states, contribute to its metallic character and semiconductor properties, and allow it to form a vast array of compounds with significant applications in various fields, including electronics, medicine, and metallurgy. Understanding the electronic structure of gallium is crucial to appreciating its versatility and its impact on modern technologies. The influence of these three electrons extends far beyond a simple number, shaping the element's behavior and its role in our world. Further research into gallium and its compounds promises even more exciting discoveries and applications in the future.
Latest Posts
Latest Posts
-
What Is 9 To The Power Of 0
Apr 02, 2025
-
Least Common Multiple Of 36 And 12
Apr 02, 2025
-
Greatest Common Factor Of 24 And 42
Apr 02, 2025
-
What 3 Particles Make Up An Atom
Apr 02, 2025
-
Blood Is What Type Of Mixture
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Gallium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
