Which Type Of Chemical Bond Involves The Exchange Of Electrons
listenit
Mar 31, 2025 · 6 min read
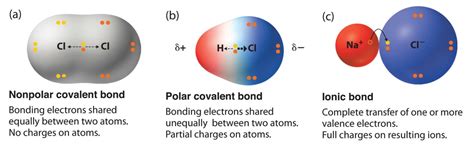
Table of Contents
Which Type of Chemical Bond Involves the Exchange of Electrons?
The answer is straightforward: ionic bonds. Ionic bonds are formed through the complete transfer of one or more electrons from one atom to another. This transfer creates ions – charged atoms – which are then held together by the electrostatic attraction between their opposite charges. Understanding ionic bonds requires delving into the fundamental principles of atomic structure and electron behavior. This article will explore ionic bonding in detail, contrasting it with other types of chemical bonds and examining its properties and applications.
Understanding Atomic Structure and Electron Behavior
Before diving into the specifics of ionic bonding, it's crucial to grasp the basics of atomic structure. Atoms consist of a nucleus containing protons (positively charged) and neutrons (neutral), surrounded by electrons (negatively charged) orbiting in various energy levels or shells. The outermost shell, known as the valence shell, plays a critical role in chemical bonding. Atoms tend to be most stable when their valence shell is either full (usually containing eight electrons, the "octet rule") or empty. This inherent drive for stability dictates how atoms interact and form bonds.
The Octet Rule and its Exceptions
The octet rule, while a useful guideline, isn't absolute. Many exceptions exist, particularly for atoms with fewer than four valence electrons or those in the third row and beyond of the periodic table. For example, elements like boron (B) often form compounds with only six electrons in their valence shell, and expanded octets (more than eight electrons) are common among elements in the third row and below. Understanding these exceptions is essential for comprehending the complexities of chemical bonding.
Ionic Bonds: A Detailed Look
Ionic bonds are formed when there's a significant difference in electronegativity between two atoms. Electronegativity measures an atom's ability to attract electrons towards itself in a chemical bond. When one atom has a significantly higher electronegativity than another, it can completely pull one or more electrons away from the less electronegative atom. This electron transfer results in the formation of ions: a positively charged cation (the atom that loses electrons) and a negatively charged anion (the atom that gains electrons). The electrostatic attraction between these oppositely charged ions constitutes the ionic bond.
Formation of Ionic Bonds: A Step-by-Step Illustration
Let's illustrate ionic bond formation with a classic example: the formation of sodium chloride (NaCl), common table salt.
-
Sodium (Na): Sodium has one electron in its valence shell. It readily loses this electron to achieve a stable, filled valence shell like that of the noble gas neon (Ne). Losing an electron leaves it with one more proton than electron, resulting in a +1 charge: Na⁺.
-
Chlorine (Cl): Chlorine has seven electrons in its valence shell. It readily gains one electron to achieve a stable octet, similar to the noble gas argon (Ar). Gaining an electron gives it one more electron than proton, resulting in a -1 charge: Cl⁻.
-
Electrostatic Attraction: The positively charged Na⁺ ion and the negatively charged Cl⁻ ion are strongly attracted to each other due to their opposite charges. This electrostatic attraction constitutes the ionic bond, forming the crystal lattice structure of NaCl.
Properties of Ionic Compounds
Ionic compounds, resulting from ionic bonds, exhibit several characteristic properties:
-
High melting and boiling points: The strong electrostatic forces between ions require a significant amount of energy to overcome, leading to high melting and boiling points.
-
Crystalline structure: Ionic compounds typically form well-ordered, crystalline structures due to the regular arrangement of ions in a three-dimensional lattice.
-
Hardness and Brittleness: Ionic crystals are relatively hard due to the strong ionic bonds. However, they are also brittle because the displacement of ions can lead to repulsion between like charges, causing the crystal to fracture.
-
Solubility in polar solvents: Ionic compounds are often soluble in polar solvents like water because the polar solvent molecules can effectively surround and solvate the ions, overcoming the electrostatic attraction between them.
-
Electrical conductivity: While solid ionic compounds are poor conductors of electricity, they conduct electricity when molten (liquid) or dissolved in water because the ions become mobile and can carry an electric current.
Comparing Ionic Bonds with Other Types of Chemical Bonds
While ionic bonds involve the complete transfer of electrons, other types of chemical bonds involve different mechanisms of electron sharing or interaction.
Covalent Bonds: Sharing is Caring
In covalent bonds, atoms share electrons to achieve a stable electron configuration. This sharing is often equal (nonpolar covalent) or unequal (polar covalent), depending on the electronegativity difference between the atoms involved. Unlike ionic bonds, there's no complete transfer of electrons.
-
Nonpolar Covalent Bonds: These occur between atoms with similar electronegativities, resulting in an even distribution of shared electrons. Examples include the bonds in diatomic molecules like O₂ and N₂.
-
Polar Covalent Bonds: These occur between atoms with different electronegativities. The shared electrons are more attracted to the atom with the higher electronegativity, creating a partial positive charge (δ+) on the less electronegative atom and a partial negative charge (δ-) on the more electronegative atom. Water (H₂O) is a classic example, with oxygen having a higher electronegativity than hydrogen.
Metallic Bonds: A Sea of Electrons
Metallic bonds are found in metals. In this type of bonding, valence electrons are delocalized, meaning they are not associated with any particular atom but rather move freely throughout the metal lattice. This "sea" of delocalized electrons accounts for the characteristic properties of metals, such as high electrical and thermal conductivity, malleability, and ductility.
Applications of Ionic Compounds
Ionic compounds have numerous applications across various fields:
-
Medicine: Many pharmaceuticals are ionic compounds, playing crucial roles in treating various ailments. For example, sodium chloride is an essential electrolyte, and numerous medications use ionic forms of active ingredients.
-
Agriculture: Ionic compounds like fertilizers provide essential nutrients to plants in soluble forms, enabling better absorption.
-
Industry: Ionic compounds are used extensively in manufacturing processes. For example, sodium hydroxide (NaOH) is a strong base used in many industrial applications.
-
Everyday life: Common table salt (NaCl), baking soda (NaHCO₃), and many other household items are ionic compounds.
Conclusion: The Importance of Ionic Bonding
Ionic bonding, the complete transfer of electrons between atoms, plays a fundamental role in the formation of a vast array of compounds with diverse properties and applications. Understanding ionic bonds is critical for comprehending chemical reactions, material properties, and the behavior of matter in various contexts. The contrasts between ionic bonding and other bonding types highlight the versatility and complexity of chemical interactions within the world around us. This knowledge forms the foundation for numerous scientific advancements and technological innovations. Further exploration into the intricacies of ionic bonding and its relationship with other bond types will continue to unlock a deeper understanding of the natural world.
Latest Posts
Latest Posts
-
How To Determine Zeros Of A Function
Apr 02, 2025
-
What Are Four Principles Of Natural Selection
Apr 02, 2025
-
What Is 9 To The Power Of 0
Apr 02, 2025
-
Least Common Multiple Of 36 And 12
Apr 02, 2025
-
Greatest Common Factor Of 24 And 42
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Type Of Chemical Bond Involves The Exchange Of Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
