What Is The Charge Of Cl
listenit
Mar 24, 2025 · 6 min read
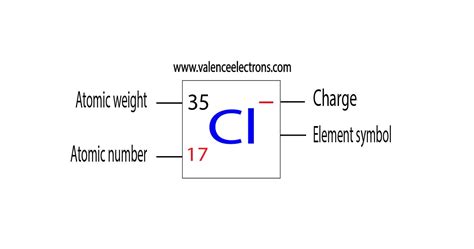
Table of Contents
What is the Charge of Cl? Understanding Chlorine's Ionic Behavior
Chlorine (Cl), a vibrant yellow-green gas, is a halogen element that plays a crucial role in various aspects of our lives, from water purification to industrial processes. Understanding its chemical behavior, particularly its charge, is essential for comprehending its reactivity and applications. This article delves deep into the charge of chlorine, exploring its ionic and covalent bonding characteristics, its oxidation states, and the implications of its charge in different chemical contexts.
Chlorine's Electronic Structure: The Key to its Charge
To understand the charge of Cl, we must first examine its electronic configuration. Chlorine's atomic number is 17, meaning it has 17 protons and 17 electrons in a neutral atom. These electrons are arranged in shells: 2 in the first shell, 8 in the second, and 7 in the outermost, or valence, shell. This outermost shell is crucial in determining chlorine's reactivity. Atoms strive for a stable electron configuration, often resembling the noble gases with their filled outer shells. For chlorine, this means achieving a full octet – eight electrons in its valence shell.
Achieving a Stable Octet: Gaining an Electron
Since chlorine has 7 valence electrons, it's easier for it to gain one electron rather than lose seven to achieve a stable octet. This electron gain results in the formation of a chloride ion (Cl⁻). The addition of an electron gives chlorine a negative charge of -1. This is the most common charge associated with chlorine.
Ionic Bonding: Chlorine's Role in Salt Formation
The tendency of chlorine to gain an electron is central to its participation in ionic bonding. Ionic bonding occurs when one atom transfers an electron to another, forming ions with opposite charges that are electrostatically attracted to each other. A classic example is the formation of sodium chloride (NaCl), common table salt.
Sodium (Na), an alkali metal, has one valence electron. It readily loses this electron to achieve a stable octet, forming a positively charged sodium ion (Na⁺). This lost electron is readily accepted by chlorine, creating the negatively charged chloride ion (Cl⁻). The electrostatic attraction between the Na⁺ and Cl⁻ ions forms the ionic bond that holds the crystal structure of sodium chloride together.
Other Ionic Compounds with Chlorine
Chlorine's ability to form ionic bonds extends beyond sodium chloride. It readily reacts with numerous other metals, forming a wide array of ionic compounds. Examples include:
- Potassium chloride (KCl): Potassium, like sodium, easily loses one electron to form a K⁺ ion, which bonds with Cl⁻.
- Magnesium chloride (MgCl₂): Magnesium loses two electrons to form Mg²⁺, requiring two chloride ions (Cl⁻) to balance the charge.
- Calcium chloride (CaCl₂): Similar to magnesium chloride, calcium (Ca) loses two electrons, forming Ca²⁺ which combines with two Cl⁻ ions.
These examples highlight chlorine's versatility in forming ionic compounds with various metals, always exhibiting a -1 charge as a chloride ion.
Covalent Bonding: Sharing Electrons with Chlorine
While ionic bonding is dominant for chlorine's interaction with metals, it also participates in covalent bonding with non-metals. Covalent bonding involves the sharing of electrons between atoms to achieve stable electron configurations. In covalent bonds involving chlorine, it typically shares one electron to complete its octet.
Examples of Covalent Compounds with Chlorine
Numerous important covalent compounds contain chlorine, including:
- Hydrogen chloride (HCl): Chlorine shares one electron with hydrogen to form a single covalent bond. This compound is a gas at room temperature and dissolves in water to form hydrochloric acid, a strong acid.
- Chloromethane (CH₃Cl): Chlorine shares one electron with a carbon atom in this organic compound. Chloromethane is used as a refrigerant and a solvent.
- Dichloromethane (CH₂Cl₂): This compound contains two chlorine atoms, each sharing one electron with a carbon atom. Dichloromethane is used as a solvent in various applications.
In these covalent compounds, chlorine doesn't carry a formal -1 charge; instead, the electrons are shared between atoms. However, because chlorine is more electronegative than carbon and hydrogen, it pulls the shared electrons closer to itself, creating a partial negative charge (δ⁻) on the chlorine atom. This difference in electronegativity leads to polar covalent bonds.
Oxidation States: Beyond the -1 Charge
While the -1 charge is the most common for chlorine, it can exhibit other oxidation states in different chemical environments. The oxidation state represents the hypothetical charge an atom would have if all bonds were completely ionic.
Variable Oxidation States
Chlorine can exhibit oxidation states ranging from -1 to +7. The -1 state is most stable, seen in ionic compounds like NaCl. Higher oxidation states are found in compounds where chlorine is bonded to more electronegative elements like oxygen. Examples include:
- Chlorate ion (ClO₃⁻): Chlorine has an oxidation state of +5.
- Perchlorate ion (ClO₄⁻): Chlorine has an oxidation state of +7.
- Chlorine dioxide (ClO₂): Chlorine has an oxidation state of +4.
These higher oxidation states indicate that chlorine can, under certain circumstances, lose or share electrons in ways that deviate from simply gaining one electron to form Cl⁻. This reflects the complex chemistry that this versatile element is capable of.
The Significance of Chlorine's Charge in Various Applications
The charge of chlorine plays a significant role in its wide range of applications. Its -1 charge as a chloride ion is vital for:
-
Water Purification: Chlorine is used extensively to disinfect water by killing harmful bacteria and viruses. The chloride ions themselves don't directly kill pathogens; it is the hypochlorous acid (HOCl) and hypochlorite ion (OCl⁻) formed when chlorine reacts with water that possess significant disinfecting power. The presence of chloride ions is also an indicator of the effectiveness of the disinfection process.
-
Industrial Processes: Chlorine is a key reactant in the production of numerous chemicals, including plastics (PVC), solvents, and pesticides. Its ability to form both ionic and covalent bonds, depending on the reaction conditions, allows it to participate in a diverse range of chemical transformations.
-
Medical Applications: Chloride ions are essential for maintaining proper fluid balance and nerve function in the human body. Hydrochloric acid (HCl), which is produced in the stomach, is crucial for digestion.
-
Household Uses: Sodium hypochlorite (NaClO), which contains chlorine with a +1 oxidation state, is the active ingredient in many household bleaches.
Conclusion: A Versatile Element with a Predominant Negative Charge
In conclusion, while chlorine's most common charge is -1 as the chloride ion (Cl⁻), formed by gaining one electron to achieve a stable octet, its versatility extends beyond this simple description. Its ability to form ionic and covalent bonds and exhibit various oxidation states highlights its critical role in a vast array of chemical processes and applications, from everyday life to sophisticated industrial applications. Understanding chlorine's electronic structure and its subsequent charge behaviors is essential for grasping its importance in chemistry and numerous fields that rely upon it. The -1 charge remains a cornerstone of understanding chlorine's chemical interactions, but the broader picture reveals a much more intricate and fascinating element.
Latest Posts
Latest Posts
-
Groups 3 12 Contain Metals Known As
Mar 25, 2025
-
How Many Atoms Are Required To Form A Molecule
Mar 25, 2025
-
What Is Terminal Side Of An Angle
Mar 25, 2025
-
How Monomers And Polymers Are Related
Mar 25, 2025
-
Elements In Group 3 12 Are Called
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of Cl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
