How Many Atoms Are Required To Form A Molecule
listenit
Mar 25, 2025 · 6 min read
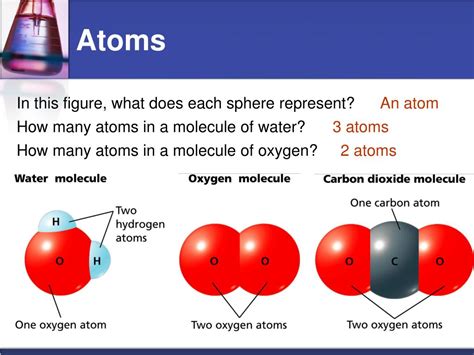
Table of Contents
How Many Atoms Are Required to Form a Molecule?
The question of how many atoms are required to form a molecule is deceptively simple. While the intuitive answer might be "two or more," a deeper understanding requires exploring the nature of chemical bonds, molecular structures, and the diverse world of chemical compounds. This article delves into these aspects, examining various scenarios and clarifying the nuances of molecular formation.
Defining Molecules: The Building Blocks of Matter
Before we delve into the number of atoms, let's precisely define a molecule. A molecule is a group of two or more atoms held together by chemical bonds. These atoms can be of the same element (like in the oxygen molecule, O₂) or different elements (like in a water molecule, H₂O). The key here is the chemical bond; it's the electrostatic force of attraction that holds the atoms together. These bonds are formed through the sharing or transfer of electrons, resulting in a stable and relatively independent entity.
Distinguishing Molecules from Atoms and Ions
It's crucial to differentiate molecules from individual atoms and ions. An atom is the fundamental unit of an element, containing a nucleus of protons and neutrons surrounded by electrons. An ion, on the other hand, is an atom or molecule that carries a net electric charge due to the loss or gain of electrons. Molecules are distinct from both; they are formed from the combination of atoms or ions, creating a new entity with its own unique properties.
The Minimum Number of Atoms: The Diatomic Case
The simplest molecules consist of two atoms, also known as diatomic molecules. Examples include:
- Oxygen (O₂): Two oxygen atoms bonded together, vital for respiration.
- Hydrogen (H₂): Two hydrogen atoms bonded, the simplest molecule.
- Nitrogen (N₂): Two nitrogen atoms bonded, forming the majority of the Earth's atmosphere.
- Chlorine (Cl₂): Two chlorine atoms bonded, a toxic gas.
- Fluorine (F₂): Two fluorine atoms bonded, highly reactive.
- Bromine (Br₂): Two bromine atoms bonded, a reddish-brown liquid.
- Iodine (I₂): Two iodine atoms bonded, a dark violet solid.
These diatomic molecules represent the minimum number of atoms needed to form a molecule – two. The atoms are held together by covalent bonds, where they share electrons to achieve a more stable electron configuration.
Beyond Diatomic: Polyatomic Molecules and Their Complexity
Moving beyond two atoms, we enter the realm of polyatomic molecules. These molecules comprise three or more atoms and exhibit a vast range of structures and properties. The number of atoms in a polyatomic molecule can range from a few to thousands, even millions, in the case of macromolecules like proteins and polymers.
Examples of polyatomic molecules include:
- Water (H₂O): Two hydrogen atoms and one oxygen atom, essential for life.
- Carbon dioxide (CO₂): One carbon atom and two oxygen atoms, a greenhouse gas.
- Ammonia (NH₃): One nitrogen atom and three hydrogen atoms, used in fertilizers.
- Methane (CH₄): One carbon atom and four hydrogen atoms, a primary component of natural gas.
- Glucose (C₆H₁₂O₆): Six carbon atoms, twelve hydrogen atoms, and six oxygen atoms, a simple sugar.
- Proteins: Complex macromolecules containing thousands of atoms, performing various biological functions.
- DNA: Extremely large molecules containing millions of atoms, carrying genetic information.
The complexity of polyatomic molecules increases dramatically with the number of atoms involved. This leads to a broader spectrum of chemical and physical properties, influencing their reactivity, solubility, and other characteristics. The arrangement of atoms (molecular geometry) also significantly impacts these properties.
Factors Influencing Molecular Formation: Bond Types and Stability
The number of atoms in a molecule is not arbitrary. It’s determined by several factors, primarily the type of chemical bonds and the stability of the resulting structure.
Covalent Bonds: Sharing is Caring
Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration, usually a full outer electron shell. The number of atoms involved depends on the number of electrons each atom can share. For instance, carbon (with four valence electrons) can form covalent bonds with up to four other atoms. This explains the abundance of carbon-based molecules in organic chemistry.
Ionic Bonds: Opposites Attract
Ionic bonds form when one atom donates an electron to another, creating ions with opposite charges that attract each other. These bonds usually lead to simpler molecules compared to those formed by covalent bonds. However, even in ionic compounds, you can have molecules like the ammonium ion (NH₄⁺). This is composed of one nitrogen atom and four hydrogen atoms bound together.
Metallic Bonds: A Sea of Electrons
Metallic bonds are found in metals and involve a "sea" of delocalized electrons shared among many metal atoms. While the concept of individual molecules is less defined in metals, the interaction between atoms is still crucial in determining their properties.
Van der Waals Forces: Weak but Important
Van der Waals forces are weak intermolecular forces that influence the behavior of molecules. They are not strong enough to form molecules but significantly impact the properties of substances, such as their boiling points and melting points.
Giant Molecules: Where the Atom Count Soars
The world of chemistry also encompasses giant molecules or macromolecules, such as polymers and biomolecules (proteins, DNA, RNA). These molecules are characterized by a vast number of atoms, often exceeding millions or even billions. Their structure and function are incredibly complex, leading to a wide array of properties and biological roles.
The size and complexity of these macromolecules are responsible for their unique characteristics. Their large size often translates into high molecular weights, influencing their physical properties like viscosity and diffusion rates. The intricate arrangement of atoms within these macromolecules also determines their biological functions.
Predicting the Number of Atoms: It's Not Always Simple
Predicting the exact number of atoms in a molecule isn't always straightforward. While simple molecules have predictable structures, larger and more complex molecules can have many possible configurations, isomers, and conformations. Determining the precise atomic composition often requires sophisticated analytical techniques, including:
- Mass spectrometry: Determining the mass-to-charge ratio of ions, providing information about molecular weight and composition.
- Nuclear magnetic resonance (NMR) spectroscopy: Analyzing the magnetic properties of atomic nuclei to determine molecular structure.
- X-ray crystallography: Determining the three-dimensional structure of molecules by analyzing diffraction patterns of X-rays.
Conclusion: A Wide Range of Molecular Sizes and Structures
In conclusion, the number of atoms required to form a molecule is at least two, as seen in diatomic molecules. However, the range extends far beyond this minimum, encompassing polyatomic molecules with varying degrees of complexity and giant molecules with millions or even billions of atoms. The factors influencing molecular formation, including bond types and stability, contribute to the diversity of molecular sizes and structures that shape our world. Understanding these fundamental principles is vital for comprehending the behavior and properties of matter.
Latest Posts
Latest Posts
-
Empirical And Molecular Formula Of Ibuprofen
Mar 26, 2025
-
What Is The Molar Mass Of So2
Mar 26, 2025
-
Electrons In The Outermost Energy Level Are Called
Mar 26, 2025
-
Express The Integral As A Limit Of Riemann Sums
Mar 26, 2025
-
How Many Lines Of Symmetry Rectangle
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are Required To Form A Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
