Electrons In The Outermost Energy Level Are Called
listenit
Mar 26, 2025 · 7 min read
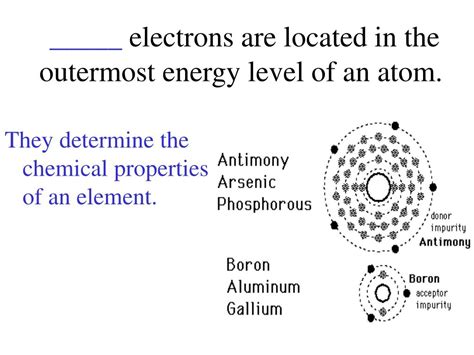
Table of Contents
Electrons in the Outermost Energy Level are Called Valence Electrons: Understanding Their Crucial Role in Chemistry
Electrons, the fundamental negatively charged particles within an atom, are not all created equal. Their location within the atom's electron cloud significantly dictates their behavior and influence on the atom's properties. Specifically, electrons residing in the outermost energy level, a region often referred to as the valence shell, hold a pivotal role in determining an element's chemical reactivity and bonding capabilities. These electrons are known as valence electrons. Understanding valence electrons is crucial to grasping the fundamental principles of chemistry and predicting how atoms will interact to form molecules and compounds.
What are Valence Electrons?
Valence electrons are the electrons located in the outermost shell or energy level of an atom. This outermost shell is often referred to as the valence shell. They are the electrons furthest from the atom's nucleus and therefore experience the weakest electrostatic attraction to the positively charged protons in the nucleus. This weaker attraction makes them significantly more likely to participate in chemical reactions and bond formation. The number of valence electrons an atom possesses directly influences its chemical behavior, determining its reactivity, the type of bonds it can form, and the number of bonds it can create.
Determining the Number of Valence Electrons
Several methods can be employed to determine the number of valence electrons an atom possesses. The most straightforward methods involve using the element's position on the periodic table or its electron configuration.
Using the Periodic Table
The periodic table is a remarkably organized chart that visually displays the elements, categorized based on their atomic structure and properties. The group number (vertical column) of an element in the periodic table directly indicates the number of valence electrons for many elements, particularly the main group elements (groups 1, 2, and 13-18).
- Groups 1 and 2: Elements in Group 1 (alkali metals) possess one valence electron, while Group 2 elements (alkaline earth metals) have two.
- Groups 13-18: For groups 13 to 18, the number of valence electrons is equal to the group number minus 10. For example, Group 13 elements have 3 valence electrons (13 - 10 = 3), Group 14 elements have 4, and so on. Group 18 elements, the noble gases, have 8 valence electrons (except for helium, which has 2).
It's important to note that this method doesn't apply directly to transition metals (elements in groups 3-12) and inner transition metals (lanthanides and actinides). These elements have more complex electron configurations, and the number of valence electrons isn't as straightforwardly determined from the group number.
Using Electron Configuration
Electron configuration describes the arrangement of electrons within an atom's energy levels and sublevels. It's a more precise method for determining the number of valence electrons, especially for elements with complex configurations. The electron configuration is represented using a notation that indicates the number of electrons in each sublevel (s, p, d, f).
For example, consider oxygen (O), which has an atomic number of 8. Its electron configuration is 1s²2s²2p⁴. The outermost shell is the second shell (n=2), and it contains 2s² and 2p⁴ electrons. Therefore, oxygen has a total of 2 + 4 = 6 valence electrons.
To identify valence electrons from the electron configuration, look at the highest principal quantum number (n) and count the electrons associated with that shell. This shell encompasses the s and p sublevels. Electrons in the d and f sublevels are generally considered inner electrons and don't directly participate in typical chemical bonding (exceptions exist).
The Significance of Valence Electrons in Chemical Bonding
Valence electrons are the primary players in chemical bonding – the process by which atoms combine to form molecules and compounds. Atoms strive for stability, often achieved by attaining a full valence shell, typically containing eight electrons (the octet rule). This tendency drives atoms to either gain, lose, or share electrons to reach a stable electron configuration.
Ionic Bonding
Ionic bonding occurs when one atom transfers one or more valence electrons to another atom. This transfer creates ions: positively charged cations (atoms that lose electrons) and negatively charged anions (atoms that gain electrons). The electrostatic attraction between these oppositely charged ions forms an ionic bond. For example, in the formation of sodium chloride (NaCl), sodium (Na) loses one valence electron to become Na⁺, and chlorine (Cl) gains this electron to become Cl⁻. The resulting electrostatic attraction holds the ions together in a crystal lattice.
Covalent Bonding
Covalent bonding occurs when atoms share valence electrons to achieve a stable electron configuration. This sharing creates a covalent bond, where the shared electrons are attracted to the nuclei of both atoms involved. For example, in a water molecule (H₂O), each hydrogen atom shares one electron with the oxygen atom, and oxygen shares two electrons, one with each hydrogen. This arrangement allows all atoms to effectively achieve a full valence shell.
Metallic Bonding
Metallic bonding occurs in metals, where valence electrons are delocalized and form a "sea" of electrons that surrounds positively charged metal ions. This sea of electrons allows for the high electrical and thermal conductivity characteristic of metals. The electrons are free to move throughout the metal structure, contributing to its malleability and ductility.
Valence Electrons and Chemical Properties
The number of valence electrons directly influences an element's chemical properties, determining its reactivity and the types of bonds it can form.
-
High Reactivity: Elements with one or seven valence electrons tend to be highly reactive. They readily participate in chemical reactions to gain or lose electrons and achieve a stable electron configuration. For example, the alkali metals (Group 1) are extremely reactive due to their single valence electron, easily lost to form a +1 ion. Halogens (Group 17) are also highly reactive, readily gaining one electron to achieve a stable octet.
-
Low Reactivity: Elements with eight valence electrons (noble gases) are generally unreactive because they already possess a stable octet. They rarely participate in chemical reactions unless under extreme conditions.
-
Predicting Bonding: The number of valence electrons helps predict the type of bonds an element will form. Elements with low electronegativity tend to lose valence electrons to form ionic bonds with elements with high electronegativity. Elements with similar electronegativities tend to share electrons to form covalent bonds.
Exceptions to the Octet Rule
While the octet rule is a useful guideline, there are exceptions where atoms do not follow the rule and achieve stability with less or more than eight electrons in their valence shell. These exceptions include:
-
Incomplete Octet: Some elements, particularly those in the second period (like beryllium and boron), can achieve stability with fewer than eight electrons in their valence shell.
-
Expanded Octet: Elements in the third period and beyond can accommodate more than eight valence electrons due to the availability of d orbitals. These elements can form compounds with expanded octets, such as phosphorus pentachloride (PCl₅) and sulfur hexafluoride (SF₆).
-
Odd Electron Molecules: Molecules with an odd number of electrons, such as nitrogen dioxide (NO₂), cannot follow the octet rule for all atoms.
Conclusion
Valence electrons are the cornerstone of chemical bonding and reactivity. Their number and arrangement determine how atoms interact to form molecules and compounds, shaping the properties of matter. By understanding valence electrons, we can unlock a deeper understanding of the fundamental principles governing chemical behavior, paving the way for exploring diverse chemical phenomena and designing new materials with specific properties. Mastering the concept of valence electrons is thus an essential step in any journey into the fascinating world of chemistry. Understanding how to determine the number of valence electrons using the periodic table or electron configuration forms the basis for predicting chemical interactions and understanding the overall structure and behavior of matter.
Latest Posts
Latest Posts
-
What Does Ate Mean In Chemistry
Mar 29, 2025
-
Standard Enthalpy Of Formation Of Ethanol
Mar 29, 2025
-
Do Parallelograms Have 4 Right Angles
Mar 29, 2025
-
Least Common Multiple Of 10 And 8
Mar 29, 2025
-
Name 3 Ways To Dissolve Something Faster
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Electrons In The Outermost Energy Level Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
