Elements In Group 3 12 Are Called
listenit
Mar 25, 2025 · 7 min read
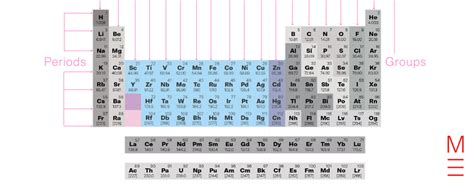
Table of Contents
Elements in Groups 3-12: A Deep Dive into Transition Metals
Elements found in groups 3 through 12 of the periodic table are collectively known as transition metals. This isn't just a label; it reflects a unique set of properties and behaviors that distinguish them from other elements. Understanding these elements is crucial for numerous fields, from materials science and engineering to medicine and catalysis. This comprehensive article will explore the characteristics, properties, and applications of these fascinating metals.
Defining Transition Metals: More Than Just a Location on the Periodic Table
The placement of transition metals in groups 3-12 isn't arbitrary. Their position reflects their electronic configuration. Unlike main group elements (groups 1, 2, and 13-18), transition metals have incompletely filled d orbitals in one or more of their oxidation states. This incomplete d subshell is the key to many of their characteristic properties. This incomplete filling allows for variable oxidation states, contributing to their diverse chemical behavior. They often form colored compounds, exhibit catalytic activity, and possess paramagnetic properties, all stemming from the intricacies of their electron configurations and the resulting bonding interactions.
Key Characteristics of Transition Metals
Several key characteristics define and distinguish transition metals:
1. Variable Oxidation States: The Chameleons of Chemistry
One of the most striking features of transition metals is their ability to exist in multiple oxidation states. This means a single transition metal atom can lose different numbers of electrons to form ions with varying charges. For example, iron (Fe) can exist as Fe²⁺ (ferrous) and Fe³⁺ (ferric), leading to a wide range of compounds with diverse properties. This versatility arises from the relatively small energy difference between the d and s electrons, making it energetically favorable for them to participate in bonding in various ways. This property is crucial in catalysis where the metal can switch between oxidation states facilitating a reaction.
2. Formation of Colored Compounds: A Visual Spectacle
The vibrant colors displayed by many transition metal compounds are a captivating phenomenon. Unlike the typically colorless compounds of main group elements, transition metal compounds often exhibit a spectrum of colors, ranging from deep blues and greens to intense reds and yellows. This is due to the d-d electron transitions. When light shines on a transition metal ion, electrons in the d orbitals can absorb specific wavelengths of light, causing the transmission of the remaining wavelengths, leading to the perceived color. The specific color depends on the metal ion, its oxidation state, and the ligands (molecules or ions) surrounding it.
3. Catalytic Activity: The Workhorses of Chemical Reactions
Transition metals and their compounds are renowned for their catalytic activity. Catalysis involves increasing the rate of a chemical reaction without being consumed in the process. Transition metals' ability to exhibit multiple oxidation states allows them to act as electron acceptors or donors during a reaction, facilitating the transformation of reactants into products. This makes them invaluable in various industrial processes, including the Haber-Bosch process for ammonia synthesis and the catalytic converters in automobiles, effectively cleaning exhaust gases.
4. Paramagnetism: A Magnetic Attraction
Many transition metals and their compounds exhibit paramagnetism, meaning they are weakly attracted to a magnetic field. This property stems from the presence of unpaired electrons in the d orbitals. The unpaired electrons possess magnetic moments that interact with the applied magnetic field, resulting in a weak attraction. This contrasts with diamagnetic materials, which are repelled by magnetic fields due to the absence of unpaired electrons. Understanding the magnetic properties of transition metals is critical in designing and developing new magnetic materials.
Individual Elements and Their Notable Applications
Exploring individual transition metals reveals a wealth of specific applications:
Titanium (Ti): Lightweight and Strong
Titanium is a lightweight yet exceptionally strong metal, valued for its high strength-to-weight ratio. This makes it ideal for aerospace applications, where weight reduction is crucial, and in biomedical implants due to its biocompatibility and resistance to corrosion.
Vanadium (V): Steel Alloying and Batteries
Vanadium is primarily used as an alloying agent in steel, improving its strength and toughness. It also finds applications in vanadium redox flow batteries, offering a promising energy storage solution for renewable energy integration.
Chromium (Cr): Corrosion Resistance and Pigments
Chromium is known for its exceptional corrosion resistance, often used as a protective coating on other metals (chromium plating). Its compounds are also used as pigments in paints and inks.
Manganese (Mn): Steel Production and Batteries
Manganese is a vital component in steel production, enhancing its hardness and strength. It also plays a critical role in lithium-ion batteries.
Iron (Fe): The Backbone of Industry
Iron is arguably the most important transition metal, forming the basis of numerous steels and alloys. Its abundance and relative ease of extraction have made it foundational to modern industrial society.
Cobalt (Co): Magnets and Catalysts
Cobalt is crucial in the production of strong permanent magnets, used in various electrical motors and devices. It's also a key component in some catalysts, particularly in the petroleum industry.
Nickel (Ni): Alloys, Catalysts, and Batteries
Nickel is extensively used in various alloys, including stainless steel, providing corrosion resistance and enhanced mechanical properties. It's also employed as a catalyst and in rechargeable batteries (Ni-Cd and Ni-MH).
Copper (Cu): Conductivity and Applications
Copper's excellent electrical conductivity makes it indispensable in electrical wiring and electronics. Its malleability and ductility also contribute to its use in plumbing and other applications.
Zinc (Zn): Galvanization and Batteries
Zinc is mainly utilized in galvanization, a process that protects steel from corrosion. It is also a key component in various batteries, including dry cell batteries.
Silver (Ag): Conductivity and Antimicrobial Properties
Silver's exceptional electrical conductivity, along with its resistance to oxidation, makes it a valuable material in electronics and jewelry. Its antimicrobial properties have led to its use in wound dressings and water purification.
Gold (Au): Inertness and Value
Gold's inertness towards most chemicals and its beautiful luster have made it a highly prized material for centuries. It's used in jewelry, electronics, and dentistry.
Mercury (Hg): Unique Liquid Metal
Mercury is a unique liquid metal at room temperature, although its toxicity has limited its applications. It was historically used in thermometers and barometers, but its use is now significantly restricted due to environmental concerns.
The Importance of Transition Metals in Modern Society
The role of transition metals in modern society is pervasive. They are essential components in:
-
Construction and Infrastructure: Steel, alloys, and other transition metal-based materials are foundational to buildings, bridges, and transportation infrastructure.
-
Electronics and Technology: Copper, gold, silver, and other transition metals are indispensable in electronic devices, computers, and communication technologies.
-
Energy Production and Storage: Transition metals play critical roles in various energy technologies, including catalysts for fuel processing and components in batteries and fuel cells.
-
Medicine and Healthcare: Transition metals are used in medical implants, diagnostic tools, and pharmaceuticals.
-
Catalysis and Chemical Industries: Transition metal catalysts are essential in numerous industrial chemical processes, affecting the production of numerous goods.
Ongoing Research and Future Prospects
Research into transition metals continues to expand, focusing on:
-
Developing new catalysts: Researchers are actively seeking more efficient and environmentally friendly catalysts based on transition metals for various chemical processes.
-
Improving battery technology: Transition metals are crucial for developing high-performance batteries for electric vehicles and energy storage applications.
-
Creating novel materials: New materials with unique properties are being developed using combinations of transition metals, opening up possibilities in various fields.
-
Understanding biological roles: The biological functions of transition metals in living organisms are an active area of research.
In conclusion, the elements in groups 3-12, the transition metals, are a fascinating and diverse group possessing unique properties that have revolutionized various aspects of modern society. Their variable oxidation states, catalytic activity, and formation of colored compounds are just some of the characteristics that make them indispensable in countless applications, continuing to drive advancements in technology and scientific understanding. Further research promises to unlock even more potential for these remarkable elements.
Latest Posts
Latest Posts
-
What Percent Of 12 5 Is 39
Mar 26, 2025
-
Empirical And Molecular Formula Of Ibuprofen
Mar 26, 2025
-
What Is The Molar Mass Of So2
Mar 26, 2025
-
Electrons In The Outermost Energy Level Are Called
Mar 26, 2025
-
Express The Integral As A Limit Of Riemann Sums
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Elements In Group 3 12 Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
