Two Or More Elements Chemically Combined
listenit
Mar 16, 2025 · 6 min read
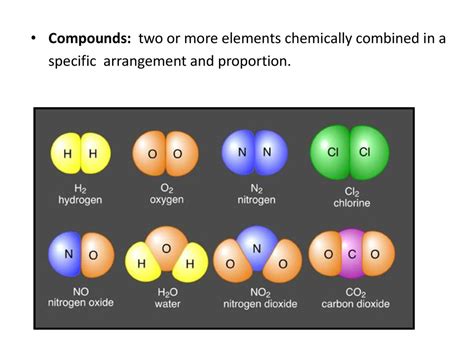
Table of Contents
Two or More Elements Chemically Combined: Delving into the World of Compounds
When two or more elements chemically combine, they form a compound. This isn't just a simple mixture, like sand and water; it's a fundamentally new substance with properties entirely different from its constituent elements. Understanding compounds is crucial to grasping the basics of chemistry, as they form the building blocks of almost everything around us – from the air we breathe to the food we eat. This article will delve into the fascinating world of compounds, exploring their formation, properties, types, and significance.
The Essence of Chemical Bonding
The key to understanding compound formation lies in chemical bonding. This is the process where atoms of elements interact and share, donate, or accept electrons to achieve a more stable electronic configuration, typically resembling that of a noble gas (Group 18 elements). There are several types of chemical bonds, each leading to distinct characteristics in the resulting compound:
1. Ionic Bonds: The Dance of Ions
Ionic bonds form when one atom transfers one or more electrons to another atom. This transfer creates ions: positively charged cations (atoms that lose electrons) and negatively charged anions (atoms that gain electrons). The electrostatic attraction between these oppositely charged ions forms the ionic bond.
Example: Sodium chloride (NaCl), common table salt, is a classic example. Sodium (Na) readily loses one electron to become a Na⁺ cation, while chlorine (Cl) readily gains one electron to become a Cl⁻ anion. The strong electrostatic attraction between these ions forms the crystalline structure of NaCl. The properties of NaCl—its high melting point, solubility in water, and ability to conduct electricity when molten or dissolved—are dramatically different from those of its constituent elements, sodium (a highly reactive metal) and chlorine (a toxic gas).
2. Covalent Bonds: Sharing is Caring
Covalent bonds form when atoms share one or more pairs of electrons. This sharing allows both atoms to achieve a stable electron configuration. Covalent bonds are typically found between nonmetal atoms.
Example: Water (H₂O) is a prime example of a covalent compound. Two hydrogen atoms each share a pair of electrons with an oxygen atom, forming two covalent bonds. The resulting molecule has unique properties: it's a liquid at room temperature, has a high specific heat capacity, and acts as a universal solvent, crucial for life as we know it. The properties of water are drastically different from hydrogen (a highly flammable gas) and oxygen (a gas essential for respiration).
3. Metallic Bonds: A Sea of Electrons
Metallic bonds occur in metals. In this type of bonding, valence electrons are delocalized, meaning they are not associated with any particular atom but are free to move throughout the metal lattice. This "sea" of delocalized electrons accounts for the characteristic properties of metals: high electrical and thermal conductivity, malleability, and ductility.
Types of Compounds: A Diverse Chemical World
The sheer variety of compounds is staggering. They can be broadly classified into several categories:
1. Inorganic Compounds: The Non-Carbon Realm
Inorganic compounds typically do not contain carbon-hydrogen bonds (although some exceptions exist). They encompass a wide range of substances, including:
- Acids: Substances that donate protons (H⁺ ions) in solution. Examples include hydrochloric acid (HCl) and sulfuric acid (H₂SO₄).
- Bases: Substances that accept protons or donate hydroxide ions (OH⁻) in solution. Examples include sodium hydroxide (NaOH) and calcium hydroxide (Ca(OH)₂)
- Salts: Ionic compounds formed from the reaction of an acid and a base. Table salt (NaCl) is a common example.
- Oxides: Compounds containing oxygen combined with another element. Examples include iron oxide (Fe₂O₃, rust) and carbon dioxide (CO₂).
2. Organic Compounds: The Carbon-Based World
Organic compounds are primarily composed of carbon and hydrogen, often in combination with oxygen, nitrogen, sulfur, and other elements. The unique bonding capabilities of carbon allow for the formation of an incredibly vast array of molecules, forming the backbone of living organisms and many synthetic materials.
- Hydrocarbons: Compounds consisting solely of carbon and hydrogen, such as methane (CH₄), ethane (C₂H₆), and benzene (C₆H₆).
- Alcohols: Compounds containing a hydroxyl group (-OH) attached to a carbon atom, such as ethanol (C₂H₅OH), the alcohol in alcoholic beverages.
- Carboxylic acids: Compounds containing a carboxyl group (-COOH), such as acetic acid (CH₃COOH), the acid in vinegar.
- Esters: Compounds formed from the reaction of a carboxylic acid and an alcohol, often responsible for the pleasant aromas of fruits and flowers.
- Amines: Compounds containing a nitrogen atom bonded to one or more carbon atoms, present in many biologically important molecules.
Properties of Compounds: A Reflection of Bonding and Structure
The properties of a compound are intimately linked to the types of bonds holding its atoms together and the arrangement of those atoms in three-dimensional space (its structure). Properties can be physical or chemical:
Physical Properties: Observable Characteristics
These are characteristics that can be observed or measured without changing the chemical composition of the substance. Examples include:
- Melting point: The temperature at which a solid changes to a liquid.
- Boiling point: The temperature at which a liquid changes to a gas.
- Solubility: The ability of a substance to dissolve in a solvent.
- Density: The mass per unit volume of a substance.
- Color: The visual appearance of a substance.
Chemical Properties: Reactivity and Transformations
These describe how a substance interacts with other substances, undergoing a change in its chemical composition. Examples include:
- Reactivity with acids: How a substance reacts when exposed to acids.
- Reactivity with bases: How a substance reacts when exposed to bases.
- Flammability: The ease with which a substance can burn.
- Toxicity: The harmful effects of a substance on living organisms.
Naming Compounds: A System of Organization
A systematic nomenclature is used to name compounds, ensuring that each compound has a unique and unambiguous name. The rules for naming vary depending on the type of compound:
- Ionic compounds: The cation is named first, followed by the anion. For example, NaCl is sodium chloride.
- Covalent compounds: Prefixes (mono-, di-, tri-, etc.) indicate the number of atoms of each element. For example, CO₂ is carbon dioxide.
- Organic compounds: A complex system of rules based on the structure and functional groups present in the molecule is employed.
Significance of Compounds: Shaping Our World
Compounds are essential to life and underpin numerous technological advancements. Here are some key areas where they play a critical role:
- Biological systems: Water, carbohydrates, proteins, lipids, and nucleic acids—all compounds—are fundamental to the structure and function of living organisms.
- Materials science: The development of new materials with specific properties relies heavily on our understanding of compounds and their behavior.
- Medicine: Many pharmaceuticals are organic compounds designed to interact with specific biological targets.
- Agriculture: Fertilizers and pesticides often involve the use of various compounds to enhance crop yields and protect against pests.
- Industry: A wide array of industrial processes utilize compounds, from the production of plastics and polymers to the refinement of metals.
Conclusion: A World of Chemical Interactions
The chemical combination of two or more elements to form compounds is a fundamental process shaping our world. Understanding the types of chemical bonds, the diverse classes of compounds, their properties, and naming conventions is crucial for grasping the intricacies of chemistry and its impact on various aspects of our lives. From the simplest molecules to the most complex biological structures, compounds hold the key to understanding the universe around us. Further exploration into specific types of compounds and their applications will undoubtedly reveal even more fascinating aspects of this expansive field.
Latest Posts
Latest Posts
-
Sum Of The Interior Angles Of A Heptagon
Mar 16, 2025
-
How To Find X Intercept From Slope Intercept Form
Mar 16, 2025
-
Inverse Function Of 1 X 2
Mar 16, 2025
-
What Is Density And Relative Density
Mar 16, 2025
-
Is Burning A Chemical Or Physical Change
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Two Or More Elements Chemically Combined . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
