What Is Density And Relative Density
listenit
Mar 16, 2025 · 6 min read
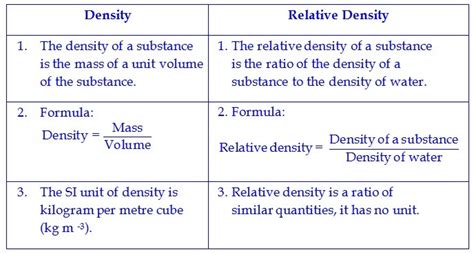
Table of Contents
What is Density and Relative Density? A Comprehensive Guide
Density and relative density are fundamental concepts in physics and material science, crucial for understanding the properties of matter and solving numerous practical problems. While often used interchangeably in casual conversation, they represent distinct but related quantities. This comprehensive guide will delve deep into the definitions, calculations, applications, and the subtle differences between density and relative density.
Understanding Density: Mass per Unit Volume
Density, often represented by the Greek letter rho (ρ), is a measure of mass per unit volume. In simpler terms, it describes how much matter is packed into a given space. A substance with high density has a lot of mass crammed into a small volume, while a substance with low density has the same mass spread over a larger volume.
Formula for Density:
The density (ρ) of a substance is calculated using the following formula:
ρ = m/V
Where:
- ρ represents density (usually measured in kg/m³ or g/cm³)
- m represents mass (usually measured in kilograms or grams)
- V represents volume (usually measured in cubic meters or cubic centimeters)
Factors Affecting Density
Several factors can influence the density of a substance:
- Temperature: Temperature changes affect the volume of a substance. Generally, as temperature increases, the volume increases (except for water between 0°C and 4°C), leading to a decrease in density. This is why hot air rises.
- Pressure: Increased pressure compresses a substance, reducing its volume and increasing its density. This effect is more pronounced in gases than in solids or liquids.
- Composition: The composition of a substance directly impacts its density. A mixture or alloy will have a density that is a weighted average of the densities of its components. For example, adding a denser element to a metal alloy increases the overall density.
- Phase: The phase of a substance (solid, liquid, or gas) significantly affects its density. Solids typically have the highest density, followed by liquids, and then gases. This is because the particles are more closely packed in solids than in liquids or gases.
Applications of Density
Understanding and utilizing density has numerous practical applications across various fields:
- Material Science: Density is crucial for material selection in engineering and manufacturing. Choosing materials with appropriate density is essential for structural integrity, weight optimization, and cost-effectiveness.
- Fluid Mechanics: Density is a key parameter in fluid dynamics, influencing buoyancy, flow behavior, and pressure distribution. Archimedes' principle, which explains buoyancy, relies directly on density differences between an object and the fluid it's submerged in.
- Geology: The density of rocks and minerals provides valuable information about their composition and origin. Density measurements are used in geological surveys to identify different rock formations and explore for resources.
- Medicine: Density measurements are used in various medical imaging techniques, such as bone density scans (DEXA scans) to assess osteoporosis risk.
- Meteorology: Air density affects weather patterns and atmospheric pressure. Variations in air density contribute to the formation of clouds, wind, and other weather phenomena.
Understanding Relative Density: Comparing Densities
Relative density, also known as specific gravity, is a dimensionless quantity that compares the density of a substance to the density of a reference substance. The reference substance is typically water at 4°C (its point of maximum density).
Formula for Relative Density:
Relative density (RD) is calculated as:
RD = ρ<sub>substance</sub> / ρ<sub>water</sub>
Where:
- RD represents relative density (a dimensionless quantity)
- ρ<sub>substance</sub> represents the density of the substance
- ρ<sub>water</sub> represents the density of water at 4°C (approximately 1000 kg/m³ or 1 g/cm³)
Significance of Relative Density
Relative density provides a convenient way to compare the densities of different substances without needing to use units. Since it's a ratio, it's independent of the units used to measure the densities of the substance and water. This makes it a valuable tool for various applications.
For example, a relative density of 2 indicates that the substance is twice as dense as water. A relative density less than 1 indicates that the substance is less dense than water and will float in water.
Applications of Relative Density
Relative density finds applications in numerous fields:
- Gemology: Relative density is a crucial property for identifying gemstones. Different gemstones have distinct relative densities, aiding in their authentication and classification.
- Hydrology: Relative density is used to measure the salinity of water bodies. Saltier water has a higher relative density than freshwater.
- Chemical Engineering: Relative density is used to determine the concentration of solutions and mixtures. Changes in relative density can indicate changes in concentration.
- Food Industry: Relative density measurements are used to monitor the quality and consistency of food products. For example, the relative density of milk can indicate its fat content.
Density vs. Relative Density: Key Differences
While both density and relative density relate to the amount of matter in a given volume, there are significant distinctions:
| Feature | Density | Relative Density |
|---|---|---|
| Definition | Mass per unit volume | Ratio of a substance's density to water's density |
| Units | kg/m³, g/cm³, lb/ft³ | Dimensionless |
| Value | Can be any positive value | Usually expressed as a decimal number |
| Reference | No inherent reference | Water at 4°C is the standard reference |
| Application | Direct measurement of mass per unit volume | Comparison of densities |
Advanced Concepts and Applications
Beyond the basics, understanding density and relative density involves exploring more advanced concepts and applications:
Density Variation with Temperature and Pressure:
The ideal gas law (PV = nRT) helps to understand density changes in gases with temperature and pressure. Liquids and solids also exhibit density changes, though typically less dramatic than gases. These variations are crucial in various industrial processes and natural phenomena.
Archimedes' Principle and Buoyancy:
Archimedes' principle states that an object submerged in a fluid experiences an upward buoyant force equal to the weight of the fluid displaced by the object. This principle is directly related to density differences between the object and the fluid. If the object's density is less than the fluid's density, it floats; otherwise, it sinks.
Density Measurement Techniques:
Various methods exist to measure density, from simple displacement methods for solids and liquids to more sophisticated techniques for gases, including pycnometry, hydrometry, and gas density meters. The choice of method depends on the substance's properties and the desired accuracy.
Applications in Material Characterization:
Density is a key parameter in material characterization, providing insights into the material's microstructure, porosity, and defects. Density measurements are used in quality control and research to assess material properties.
Density and Phase Transitions:
Density changes dramatically during phase transitions (e.g., melting, boiling). This change is exploited in various industrial processes and in understanding natural phenomena such as the formation of ice.
Density Gradient Separation:
Density gradient separation techniques utilize variations in density to separate mixtures of particles or cells based on their density differences. This is a powerful technique used in various scientific and industrial applications.
Conclusion
Density and relative density are fundamental concepts with widespread applications across various scientific and engineering disciplines. While density is a direct measure of mass per unit volume, relative density provides a convenient comparison of a substance's density to a reference substance, usually water. Understanding both concepts is crucial for comprehending the physical properties of matter and solving numerous practical problems. The advanced concepts and applications discussed above further highlight their significance in diverse fields. This comprehensive guide provides a solid foundation for anyone seeking to understand and apply these essential concepts.
Latest Posts
Latest Posts
-
If It Takes 42 Minutes To Load 3 1 2
Mar 16, 2025
-
Lowest Common Multiple Of 16 And 20
Mar 16, 2025
-
Reaction Of Sodium Hydroxide And Calcium Chloride
Mar 16, 2025
-
Liquid Sodium Is Being Considered As An Engine Coolant
Mar 16, 2025
-
What Element Has The Largest Atomic Radius
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about What Is Density And Relative Density . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
