What Element Has The Largest Atomic Radius
listenit
Mar 16, 2025 · 5 min read
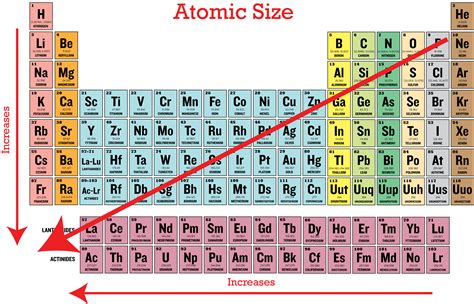
Table of Contents
What Element Has the Largest Atomic Radius? A Deep Dive into Atomic Structure and Periodic Trends
The question of which element boasts the largest atomic radius might seem simple at first glance, but delving into it reveals a fascinating exploration of atomic structure, periodic trends, and the nuances of quantum mechanics. While a straightforward answer exists, understanding why that element holds this distinction requires a closer look at the forces at play within atoms.
Understanding Atomic Radius
Before we pinpoint the champion, let's define our terms. Atomic radius isn't a precisely measurable quantity like, say, the diameter of a marble. Instead, it represents the average distance between the nucleus and the outermost electron shell of an atom. This is a tricky concept because electrons don't orbit the nucleus in neat, predictable paths as the Bohr model might suggest. Instead, they occupy orbitals, regions of space with varying probabilities of finding an electron. Therefore, atomic radius is often defined as half the distance between the nuclei of two identical atoms bonded together.
Factors Influencing Atomic Radius
Several key factors influence the size of an atom's radius:
-
Principal Quantum Number (n): As you move down a group (column) in the periodic table, the value of n increases. This means the electron shells are further away from the nucleus, resulting in a larger atomic radius. Each successive shell represents a significantly larger orbital volume.
-
Effective Nuclear Charge (Z<sub>eff</sub>): This is the net positive charge experienced by the outermost electrons. It's the difference between the actual nuclear charge (number of protons) and the shielding effect of inner electrons. A higher effective nuclear charge pulls the outer electrons closer, shrinking the atomic radius. Shielding is crucial; inner electrons effectively screen the outer electrons from the full positive charge of the nucleus.
-
Electron-Electron Repulsion: As the number of electrons increases within a shell, the repulsion between them increases. This repulsion counteracts the attractive force of the nucleus, causing the atom to expand slightly.
-
Number of Protons: A higher number of protons in the nucleus increases the positive charge, attracting electrons more strongly and thus reducing the atomic radius.
Periodic Trends and Atomic Radius
Understanding how atomic radius changes across the periodic table is vital. Two key trends emerge:
Trend 1: Across a Period (Left to Right)
As we move across a period from left to right, the atomic radius generally decreases. This is primarily due to the increasing effective nuclear charge. While additional electrons are added to the same principal energy level, the increase in nuclear charge (more protons) outweighs the increased electron-electron repulsion. The stronger nuclear pull draws the electrons closer, making the atom smaller.
Trend 2: Down a Group (Top to Bottom)
As we move down a group, the atomic radius generally increases. This is because electrons are added to successively higher principal energy levels (n increases). The increased distance from the nucleus significantly outweighs the increase in nuclear charge, resulting in a larger atomic radius.
The Element with the Largest Atomic Radius: Cesium (Cs)
Considering the trends discussed above, the element with the largest atomic radius is Cesium (Cs). Its location in the periodic table – the bottom of Group 1 (alkali metals) – is key to its size. It possesses a very high principal quantum number for its outermost electrons, placing them far from the nucleus. While the nuclear charge is significant, the shielding effect of the inner electrons and the vast distance of the outer electrons reduce the effective nuclear charge felt by those outermost electrons. This combination of factors results in a very large atomic radius.
Comparing Cesium to Other Alkali Metals and Beyond
Let's compare Cesium to its alkali metal brethren:
- Lithium (Li): Smallest alkali metal due to its low principal quantum number.
- Sodium (Na): Larger than Lithium, reflecting the increase in the principal quantum number.
- Potassium (K): Larger than Sodium, showing the continuing trend of increasing radius down the group.
- Rubidium (Rb): Larger than Potassium.
- Cesium (Cs): Largest of the alkali metals, due to the very high principal quantum number.
- Francium (Fr): While Francium technically sits below Cesium, its extreme radioactivity and short half-life make precise measurements of its atomic radius exceptionally challenging. Theoretical calculations suggest it might be slightly larger than Cesium, but the experimental uncertainty is substantial.
Beyond the alkali metals, elements in other groups with high principal quantum numbers might seem contenders for the largest atomic radius, but the balance of effective nuclear charge and electron shell distance consistently favors Cesium among the elements with experimentally verifiable data.
The Nuances of Measurement and Theoretical Calculations
It's crucial to acknowledge the inherent complexities in determining atomic radius. Different methods of measurement yield slightly different results. Furthermore, the theoretical calculations themselves rely on sophisticated quantum mechanical models which contain inherent approximations. Therefore, the precise value of Cesium's atomic radius isn't a single, definitively fixed number. However, the overall trend and the consensus across various measurement and computational techniques consistently point to Cesium as having the largest atomic radius among the elements with readily available data.
Applications and Significance
Understanding atomic radius is far from a purely academic pursuit. It has significant implications in various fields:
-
Chemistry: Atomic radius plays a crucial role in determining chemical bonding lengths and bond energies. It influences the reactivity and properties of elements and compounds. The size of an atom directly impacts its ability to interact with other atoms, affecting everything from the strength of metallic bonds to the formation of ionic and covalent bonds.
-
Material Science: The atomic radius of constituent elements is critical in designing and understanding the properties of materials. For instance, it affects the crystal structure of solids and the resulting mechanical, electrical, and thermal characteristics.
-
Nuclear Physics: Atomic radius is relevant in understanding nuclear interactions and reactions.
-
Nanotechnology: At the nanoscale, atomic radius becomes especially important. Precise control over the size and arrangement of atoms is essential for developing new nanomaterials with tailored properties.
Conclusion: Cesium Reigns Supreme (for Now)
In conclusion, while experimental complexities and theoretical limitations exist, the current understanding of atomic structure and periodic trends firmly places Cesium (Cs) as the element with the largest experimentally verifiable atomic radius. Its position in the periodic table, combined with the factors influencing atomic size, makes it the undisputed champion amongst readily studied elements. The ongoing research and refinement of quantum mechanical models will continue to offer a more precise understanding of atomic properties, but for now, Cesium holds its crown.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 5 And 6
Mar 16, 2025
-
What Are The Common Factors Of 36 And 90
Mar 16, 2025
-
Find Polar Coordinates Of The Point That Has Rectangular Coordinates
Mar 16, 2025
-
What Is A 35 Out Of 50
Mar 16, 2025
-
Difference Between Interval And Set Notation
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about What Element Has The Largest Atomic Radius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
