Is Burning A Chemical Or Physical Change
listenit
Mar 16, 2025 · 6 min read
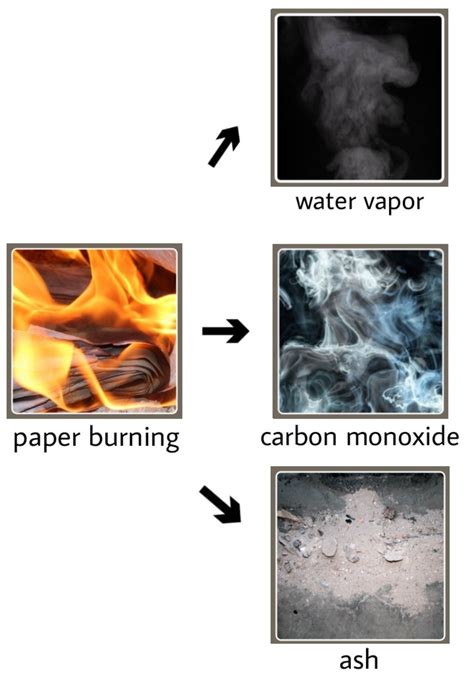
Table of Contents
Is Burning a Chemical or Physical Change? A Deep Dive into Combustion
The question of whether burning is a chemical or physical change is a fundamental one in science, often sparking debate among students and enthusiasts alike. While seemingly simple, the answer requires a nuanced understanding of both chemical and physical changes, and the complex process of combustion itself. This comprehensive article will delve into the intricacies of burning, exploring the evidence that definitively classifies it as a chemical change, and examining the various aspects that contribute to this conclusion.
Understanding Chemical and Physical Changes
Before diving into the specifics of burning, it's crucial to establish a clear understanding of the difference between chemical and physical changes. A physical change alters the form or appearance of a substance without changing its chemical composition. Think of melting ice – it changes from a solid to a liquid, but it remains H₂O. The molecules themselves are unchanged.
Conversely, a chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules to form new substances with different properties. This rearrangement often involves the breaking and forming of chemical bonds, resulting in a completely different substance than the starting material. Examples include rusting (iron reacting with oxygen) and baking a cake (ingredients chemically reacting to form a new product).
The Evidence: Why Burning is a Chemical Change
Several key pieces of evidence unequivocally demonstrate that burning is a chemical change:
1. Formation of New Substances
Burning, or combustion, is a rapid chemical reaction between a substance (the fuel) and an oxidant (usually oxygen), which produces heat and light. The products of this reaction are fundamentally different from the reactants. For example, when wood burns, it doesn't simply transform into smaller pieces of wood; it transforms into ash, smoke, carbon dioxide (CO₂), and water vapor (H₂O). These are entirely new substances with distinct physical and chemical properties compared to the original wood. This creation of entirely new substances is the hallmark of a chemical change.
2. Irreversibility
Physical changes are often reversible. For example, melting ice can be reversed by freezing the water. However, burning is largely irreversible. You cannot easily revert ash, smoke, CO₂, and water vapor back into the original piece of wood. This irreversibility is a strong indicator of a chemical reaction. While some components might be theoretically reclaimed through complex chemical processes, the original form and properties are lost permanently.
3. Energy Changes: Exothermic Reactions
Burning is an exothermic reaction, meaning it releases energy in the form of heat and light. This energy release is a direct consequence of the breaking and forming of chemical bonds during the combustion process. The energy stored within the chemical bonds of the fuel is transformed into thermal and light energy. Significant energy changes are commonly associated with chemical reactions, unlike most physical changes where energy changes are less dramatic.
4. Change in Chemical Properties
The products of burning exhibit entirely different chemical properties compared to the original fuel. For example, the wood's ability to support combustion is lost in the ash. The ash is non-combustible. Similarly, the chemical composition of smoke is vastly different from the wood's original composition, demonstrating a change in its fundamental chemical properties. This altered chemical nature is a defining characteristic of chemical change.
5. Gas Production
Many combustion processes produce gases, such as carbon dioxide and water vapor. The formation of these gases is further evidence of a chemical change, as the original fuel was not in a gaseous state before ignition. The release of gases, often visible as smoke, is a clear indication of a chemical transformation within the material being burned.
Types of Combustion and Their Chemical Changes
The specific chemical changes that occur during burning vary depending on the type of fuel and the conditions of combustion. Let's look at some examples:
1. Complete Combustion
Complete combustion occurs when there is ample oxygen available for the reaction. This typically leads to the formation of carbon dioxide and water as the primary products. For example, the complete combustion of methane (CH₄), the primary component of natural gas, can be represented by the following equation:
CH₄ + 2O₂ → CO₂ + 2H₂O + Heat + Light
Notice how the methane and oxygen molecules are rearranged to form entirely new molecules of carbon dioxide and water.
2. Incomplete Combustion
When there is insufficient oxygen, incomplete combustion occurs. This results in the formation of carbon monoxide (CO), soot (carbon particles), and other partially oxidized products. Incomplete combustion is less efficient and produces harmful pollutants. The chemical changes are still significant, but the specific products vary depending on the amount of oxygen present and the nature of the fuel.
3. Combustion of Different Fuels
The chemical changes during combustion also vary depending on the fuel source. For example, the combustion of propane (C₃H₈) produces different quantities of CO₂ and H₂O compared to the combustion of gasoline, which is a complex mixture of hydrocarbons. Each fuel has its unique chemical composition and thus reacts differently with oxygen, leading to a unique set of products and associated energy changes.
Differentiating Burning from Other Processes
It's essential to distinguish burning (combustion) from other processes that might seem similar but are fundamentally different:
- Evaporation: Evaporation is a physical change where a liquid transforms into a gas without altering its chemical composition. Water evaporating from a puddle remains water, simply changing its physical state.
- Melting: Similar to evaporation, melting is a physical change involving a change of state from solid to liquid without changing the chemical identity of the substance.
- Sublimation: Sublimation involves the direct transition from a solid to a gas without passing through the liquid phase, again a physical change.
All these processes involve changes in physical state, but not in chemical composition. In contrast, burning, with its formation of new substances, energy release, and irreversibility, clearly falls into the category of chemical change.
Conclusion: The Irrefutable Chemical Nature of Burning
The evidence presented overwhelmingly supports the conclusion that burning is a chemical change. The formation of new substances, irreversibility, significant energy changes, altered chemical properties, gas production, and the various types of combustion reactions all point towards a fundamental alteration in the chemical composition of the fuel. While seemingly a simple question, understanding the complexities of combustion provides a deeper appreciation for the fundamental principles of chemical reactions and their impact on the world around us. The next time you see a flame, remember that you are witnessing a dramatic and irreversible chemical transformation.
Latest Posts
Latest Posts
-
Where Is The Mass Of An Atom Located
Mar 16, 2025
-
How To Graph Y 1 2x 1
Mar 16, 2025
-
How Many Electrons Can Fit In The Second Shell
Mar 16, 2025
-
What Is 17 As A Decimal
Mar 16, 2025
-
If It Takes 42 Minutes To Load 3 1 2
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Is Burning A Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
