How Many Electrons Can Fit In The Second Shell
listenit
Mar 16, 2025 · 6 min read
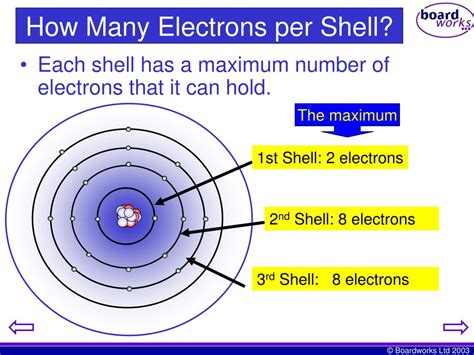
Table of Contents
How Many Electrons Can Fit in the Second Shell? A Deep Dive into Atomic Structure
Understanding the arrangement of electrons within an atom is fundamental to grasping the principles of chemistry and physics. This article delves deep into the question: how many electrons can fit in the second electron shell? We'll explore the underlying principles of electron configuration, the quantum mechanical model, and the implications of electron shell filling for chemical reactivity and the periodic table.
Understanding Electron Shells and Subshells
Atoms are composed of a nucleus containing protons and neutrons, surrounded by a cloud of negatively charged electrons. These electrons don't orbit the nucleus in neat, predictable paths like planets around a star. Instead, they exist in regions of space called electron shells or energy levels. These shells are characterized by their principal quantum number, n, which is a positive integer (1, 2, 3, and so on). The shell with n = 1 is closest to the nucleus, followed by n = 2, n = 3, and so forth. The higher the value of n, the greater the energy of the electrons in that shell and the farther they are from the nucleus.
Each electron shell isn't a uniform blob; it's further subdivided into subshells, which are designated by letters: s, p, d, and f. These subshells represent different regions of space within the shell where electrons are most likely to be found. Each subshell can hold a specific number of electrons:
- s subshell: Holds a maximum of 2 electrons.
- p subshell: Holds a maximum of 6 electrons.
- d subshell: Holds a maximum of 10 electrons.
- f subshell: Holds a maximum of 14 electrons.
The Second Electron Shell: A Detailed Look
Now, let's focus on the second electron shell, where n = 2. This shell contains two subshells: the 2s subshell and the 2p subshell.
The 2s Subshell
The 2s subshell, like all s subshells, is spherically symmetrical around the nucleus. It can hold a maximum of two electrons. These electrons have slightly different energies due to subtle interactions, but for our purposes, we can consider them to be in the same energy level.
The 2p Subshell
The 2p subshell is more complex. It consists of three orbitals, each capable of holding two electrons. These orbitals are not spherically symmetrical; instead, they have a dumbbell shape, oriented along the x, y, and z axes. The three 2p orbitals are often designated as 2p<sub>x</sub>, 2p<sub>y</sub>, and 2p<sub>z</sub>. Because each of these three orbitals can hold two electrons (one spin up and one spin down, due to the Pauli Exclusion Principle), the 2p subshell can hold a total of six electrons.
Calculating the Total Electron Capacity of the Second Shell
To determine the total number of electrons that can fit in the second shell, we simply add the maximum number of electrons in each of its subshells:
Total electrons in the second shell = electrons in 2s + electrons in 2p = 2 + 6 = 8 electrons
Therefore, the second electron shell can accommodate a maximum of eight electrons.
The Significance of the Octet Rule
The fact that the second shell holds eight electrons is crucial in chemistry because it relates directly to the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their outermost shell (valence shell). Elements in the second row of the periodic table (lithium to neon) illustrate this rule perfectly. They strive to achieve a full second shell, leading to their distinct chemical properties.
For example, oxygen (atomic number 8) has six electrons in its second shell. To achieve an octet, it readily forms two covalent bonds, sharing two pairs of electrons with other atoms. This allows it to complete its second shell and achieve greater stability. Similarly, chlorine (atomic number 17) has seven electrons in its outermost (third) shell. It readily gains one electron to complete its outermost shell (achieving an octet-like state) and form a stable chloride ion (Cl⁻).
Beyond the Second Shell: Electron Configuration and the Periodic Table
The principles governing the filling of the second shell extend to higher shells, though the details become more complex. The third shell (n = 3) contains the 3s, 3p, and 3d subshells, accommodating a total of 18 electrons. The fourth shell and beyond follow a similar pattern, with increasing numbers of subshells and electrons.
The periodic table itself reflects the filling of electron shells and subshells. Elements within the same group (column) have similar electron configurations in their outermost shell, which explains their similar chemical behaviors. For instance, all elements in Group 18 (noble gases) have a completely filled outermost shell, making them extremely unreactive.
Quantum Mechanical Considerations: The Pauli Exclusion Principle and Hund's Rule
The ability of the second shell (and other shells) to accommodate a specific number of electrons is governed by fundamental principles of quantum mechanics:
-
The Pauli Exclusion Principle: This principle states that no two electrons in an atom can have the same set of four quantum numbers. This means each orbital can hold a maximum of two electrons with opposite spins.
-
Hund's Rule: This rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to a more stable configuration.
These rules dictate how electrons fill the subshells within each shell, leading to the specific electron configurations observed in various atoms and explaining their chemical properties.
Applications and Further Exploration
The understanding of electron shell filling has numerous applications:
-
Predicting chemical reactivity: Knowing the electron configuration of an atom allows us to predict its reactivity. Atoms with incomplete outer shells tend to be more reactive than those with full outer shells.
-
Spectroscopy: The absorption and emission of light by atoms are directly related to the energy levels of their electrons and the transitions between them. Studying these spectral lines provides valuable information about atomic structure.
-
Materials science: The properties of materials (conductivity, magnetism, etc.) are strongly influenced by the electron configurations of their constituent atoms.
-
Nuclear chemistry: Understanding electron shell structure is crucial for understanding the behavior of radioactive isotopes and their decay processes.
This article provides a comprehensive overview of the electron capacity of the second shell, tying it to broader concepts in atomic structure and chemistry. Further exploration into quantum mechanics and advanced chemical principles can provide a deeper understanding of these fundamental concepts. By understanding the arrangement of electrons, we unlock the secrets of atomic behavior and the diversity of the chemical world.
Latest Posts
Latest Posts
-
What Is 8 Percent Of 1000
Mar 17, 2025
-
How Many Oz Is 2 2 Liters
Mar 17, 2025
-
How Do You Find The Magnitude Of Displacement
Mar 17, 2025
-
How Many Neutrons Does Ag Have
Mar 17, 2025
-
Why Dont Plant Cells Burst When Water Enters Them
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can Fit In The Second Shell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
