Where Is The Mass Of An Atom Located
listenit
Mar 16, 2025 · 5 min read
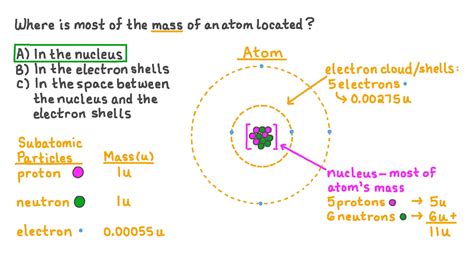
Table of Contents
Where is the Mass of an Atom Located? Delving into Atomic Structure
The seemingly simple question, "Where is the mass of an atom located?" opens a fascinating window into the complex world of atomic structure and quantum mechanics. While the answer might seem straightforward at first glance, a deeper understanding requires exploring the subatomic particles that constitute an atom and their relative contributions to its overall mass. This article will journey through the intricacies of atomic composition, highlighting the role of protons, neutrons, and electrons in determining an atom's mass and dispelling common misconceptions.
The Atomic Model: A Brief Overview
Before diving into the mass distribution, let's refresh our understanding of the atomic model. Atoms, the fundamental building blocks of matter, are composed of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus.
- Neutrons: Neutrally charged particles also found within the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The nucleus, the atom's central core, contains virtually all of its mass. This is a crucial point to grasp when considering where the mass is located. The electrons, while contributing to the atom's overall properties and chemical behavior, possess negligible mass compared to the nucleus.
The Nucleus: The Mass Heavyweight Champion
The vast majority of an atom's mass is concentrated in its nucleus. This tiny, dense region contains both protons and neutrons, collectively known as nucleons. The mass of a proton is approximately 1.67 x 10^-27 kg, while the mass of a neutron is only slightly larger, around 1.69 x 10^-27 kg. These masses are incredibly small, but they are significant when compared to the mass of an electron, which is approximately 9.11 x 10^-31 kg – nearly 2000 times smaller!
Proton Mass vs. Neutron Mass: A Subtle Difference
While often treated as having similar masses, protons and neutrons exhibit a tiny mass difference. This seemingly insignificant discrepancy plays a crucial role in certain nuclear reactions and processes, such as beta decay. However, for the purpose of understanding the overall mass distribution within an atom, this difference can be largely disregarded. The combined mass of protons and neutrons overwhelmingly dominates the total atomic mass.
Isotopes and Atomic Mass: A Deeper Dive
Atoms of the same element always have the same number of protons (this defines the atomic number), but they can have varying numbers of neutrons. These variations are called isotopes. For example, carbon-12 (¹²C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. The different numbers of neutrons lead to variations in the atomic mass of isotopes. The atomic mass number (A) represents the total number of protons and neutrons in the nucleus (A = Z + N, where Z is the atomic number and N is the number of neutrons).
The atomic weight (or relative atomic mass) listed on the periodic table is a weighted average of the masses of all naturally occurring isotopes of an element. This considers the abundance of each isotope in nature.
Electrons: The Negligible Mass Contributors
While electrons play a vital role in chemical bonding and determining an atom's chemical properties, their contribution to the overall mass is essentially negligible. The mass of an electron is so small that it can be safely ignored when calculating an atom's total mass for most practical purposes. This is why we focus almost exclusively on the nucleus when determining where the mass of an atom is located.
The Quantum Mechanical Perspective: Probability Clouds
The classical model of the atom, with electrons orbiting the nucleus in defined paths, is a simplified representation. According to quantum mechanics, electrons exist as probability clouds or orbitals, regions of space where the probability of finding an electron is high. These orbitals don't have sharply defined boundaries, making the concept of "location" for electrons more nuanced. However, even with this probabilistic description, the overwhelming majority of an atom's mass remains concentrated within the nucleus.
Implications of Mass Distribution: Nuclear Reactions and Stability
The concentration of mass within the nucleus has profound implications for various processes, especially in nuclear physics. Nuclear reactions, such as fission and fusion, involve changes within the nucleus itself, leading to significant energy releases. The stability of an atom's nucleus is determined by the balance between the strong nuclear force, which holds nucleons together, and the electromagnetic force, which repels positively charged protons. Isotopes with an unstable nucleus undergo radioactive decay, emitting particles or energy to reach a more stable configuration.
Common Misconceptions and Clarifications
Several misconceptions surrounding the location of an atom's mass are prevalent:
- The Atom is Mostly Empty Space: While the space occupied by the electrons is vast compared to the nucleus's size, this does not mean the atom is mostly empty. The density within the nucleus is exceptionally high, and its mass dominates the overall atomic mass.
- Electrons Contribute Significantly to Mass: As discussed earlier, electron mass is virtually insignificant compared to the combined mass of protons and neutrons.
- The Atomic Mass is Simply the Sum of Proton and Neutron Masses: This is a simplification. The actual atomic mass is slightly less than the sum of the individual masses due to the mass-energy equivalence (E=mc²) and the binding energy that holds the nucleus together.
Conclusion: The Nucleus Reigns Supreme
In conclusion, the overwhelming majority of an atom's mass resides in its nucleus, which houses the protons and neutrons. While electrons are crucial for an atom's chemical properties and behavior, their contribution to the overall mass is negligible. Understanding this fundamental principle of atomic structure is key to comprehending various phenomena in physics and chemistry, from nuclear reactions to the behavior of elements and compounds. The seemingly simple question of mass location unveils the intricate beauty and complexity of the atomic world. The accurate representation of where an atom's mass is located significantly impacts our understanding of various scientific fields and their related applications. Further exploration into this area continues to contribute to advancements in nuclear energy, materials science, and many other fields.
Latest Posts
Latest Posts
-
Why Dont Plant Cells Burst When Water Enters Them
Mar 17, 2025
-
Highest Common Factor Of 42 And 56
Mar 17, 2025
-
How Many Ounces Is 0 2 Pounds
Mar 17, 2025
-
Highest Human Body Temperature Ever Recorded
Mar 17, 2025
-
Does Translation Occur In The Nucleus
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Where Is The Mass Of An Atom Located . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
