The Coefficients In A Chemical Equation Represent The
listenit
Mar 29, 2025 · 5 min read
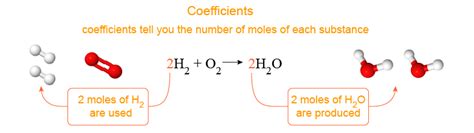
Table of Contents
The Coefficients in a Chemical Equation Represent the Moles of Reactants and Products
Understanding chemical equations is fundamental to grasping the principles of chemistry. These equations aren't just symbolic representations; they provide quantitative information about the relationships between reactants and products in a chemical reaction. A key aspect of understanding these equations lies in comprehending the significance of the coefficients. This article delves into the crucial role coefficients play, explaining precisely what they represent and how they are used in various chemical calculations.
What are Coefficients in a Chemical Equation?
Before diving into their meaning, let's define what coefficients are. In a balanced chemical equation, coefficients are the numbers placed in front of chemical formulas. They indicate the relative amounts of each substance involved in the reaction. For instance, in the equation:
2H₂ + O₂ → 2H₂O
The numbers 2, 1 (implied), and 2 are the coefficients. They are crucial for ensuring the equation is balanced, meaning that the number of atoms of each element is the same on both the reactant and product sides. It's crucial to remember that coefficients are not subscripts. Subscripts indicate the number of atoms of a particular element within a molecule.
Coefficients Represent Moles
The most significant aspect of understanding coefficients is that they represent the relative number of moles of each reactant and product involved in the reaction. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of particles (atoms, molecules, ions, etc.).
Therefore, the balanced equation 2H₂ + O₂ → 2H₂O tells us that:
- Two moles of hydrogen gas (H₂) react with
- One mole of oxygen gas (O₂) to produce
- Two moles of water (H₂O)
This is a crucial concept. Coefficients don't simply tell us about the number of molecules; they provide a macroscopic perspective, indicating the molar ratios involved.
Using Coefficients for Stoichiometric Calculations
The power of coefficients lies in their ability to facilitate stoichiometric calculations. Stoichiometry is the quantitative study of the reactants and products in chemical reactions. Coefficients provide the foundation for calculating:
-
Moles of reactants needed to produce a specific amount of product: If we need to produce 4 moles of water, we can use the coefficients to determine that we'll need 4 moles of H₂ and 2 moles of O₂.
-
Moles of product formed from a given amount of reactant: If we start with 3 moles of H₂, we can calculate the maximum amount of water that can be produced (3 moles of H₂ will produce 3 moles of H₂O).
-
Limiting reactants: In many reactions, one reactant is completely consumed before the others. This is the limiting reactant, and it determines the maximum amount of product that can be formed. Coefficients allow us to identify the limiting reactant.
-
Theoretical yield and percent yield: The theoretical yield is the maximum amount of product that can be produced based on stoichiometric calculations. The percent yield compares the actual yield (the amount of product actually obtained) to the theoretical yield. Coefficients are essential for determining the theoretical yield.
Examples of Stoichiometric Calculations
Let's consider a more complex example: The combustion of propane (C₃H₈)
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
This equation tells us that one mole of propane reacts with five moles of oxygen to produce three moles of carbon dioxide and four moles of water.
Example 1: Determining moles of product from a given amount of reactant.
If we combust 2.5 moles of propane, how many moles of carbon dioxide are produced?
Using the coefficients:
(2.5 moles C₃H₈) x (3 moles CO₂ / 1 mole C₃H₈) = 7.5 moles CO₂
Example 2: Determining moles of reactant needed to produce a specific amount of product.
How many moles of oxygen are needed to produce 10 moles of water?
Using the coefficients:
(10 moles H₂O) x (5 moles O₂ / 4 moles H₂O) = 12.5 moles O₂
Example 3: Identifying the Limiting Reactant
Let's say we have 2 moles of propane and 12 moles of oxygen. Which is the limiting reactant?
First, determine the moles of oxygen needed to react completely with 2 moles of propane:
(2 moles C₃H₈) x (5 moles O₂ / 1 mole C₃H₈) = 10 moles O₂
Since we only have 12 moles of oxygen, and we need 10 moles, oxygen is in excess. Propane is the limiting reactant. The amount of CO₂ produced will be determined by the amount of propane.
Beyond Simple Stoichiometry: Advanced Applications
The significance of coefficients extends beyond simple molar ratios. They're vital in several advanced applications:
1. Gas Stoichiometry:
Coefficients are crucial when dealing with gases because they allow for the conversion between moles and volumes using the ideal gas law (PV = nRT). The coefficients directly relate the volumes of gases involved in a reaction at constant temperature and pressure.
2. Solution Stoichiometry:
In reactions involving solutions, coefficients are used in conjunction with molarity (moles per liter) to calculate the volumes of solutions needed for a specific reaction.
3. Thermochemistry:
Coefficients are critical for interpreting thermochemical equations, which show the enthalpy change (heat released or absorbed) during a reaction. The enthalpy change is often expressed per mole of reaction, and the coefficients define what constitutes "one mole of reaction."
4. Equilibrium Calculations:
In equilibrium calculations, coefficients are incorporated into the equilibrium constant expression (K), influencing the equilibrium concentrations of reactants and products.
The Importance of Balanced Equations
It's impossible to overemphasize the importance of having a balanced chemical equation before attempting any stoichiometric calculation. Unbalanced equations will yield inaccurate results, leading to errors in experiments and predictions. Balancing equations ensures the law of conservation of mass is obeyed—the total mass of the reactants equals the total mass of the products.
Conclusion
The coefficients in a balanced chemical equation are far more than just numbers; they represent the relative number of moles of reactants and products. This fundamental understanding is crucial for accurate stoichiometric calculations, enabling chemists to predict and control the outcomes of chemical reactions. Their significance extends to various advanced chemical concepts, from gas stoichiometry to equilibrium calculations and thermochemistry. Mastering the meaning and application of coefficients is essential for success in chemistry. Remember that practice is key: work through numerous examples to solidify your understanding and build confidence in solving various stoichiometric problems. This foundational understanding will serve as a strong basis for your further studies in chemistry.
Latest Posts
Latest Posts
-
Chance Of Rolling Two Nat 20s
Mar 31, 2025
-
Does A Gas Have A Definite Shape And Volume
Mar 31, 2025
-
Factor X 3 X 2 2
Mar 31, 2025
-
How Many Neutrons Does Uranium 238 Have
Mar 31, 2025
-
Does Ccl4 Have Dipole Dipole Forces
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about The Coefficients In A Chemical Equation Represent The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
