Does A Gas Have A Definite Shape And Volume
listenit
Mar 31, 2025 · 6 min read
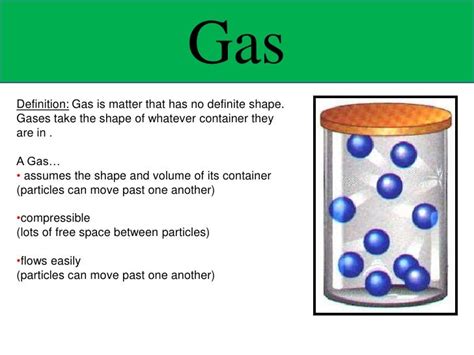
Table of Contents
Does a Gas Have a Definite Shape and Volume? Exploring the Properties of Gases
Gases, one of the fundamental states of matter, are characterized by their unique properties. Unlike solids and liquids, gases don't possess a definite shape or volume. This fundamental difference stems from the nature of the intermolecular forces and the kinetic energy of gas particles. Understanding this characteristic is crucial in various scientific fields, from chemistry and physics to meteorology and engineering. This article will delve deep into the properties of gases, exploring why they lack a defined shape and volume, and examining the factors influencing their behavior.
The Kinetic Molecular Theory: The Key to Understanding Gas Behavior
The kinetic molecular theory (KMT) provides a robust framework for understanding the behavior of gases. This theory postulates that gases consist of a large number of tiny particles (atoms or molecules) that are in constant, random motion. These particles are considered to be far apart compared to their size, meaning that the volume occupied by the particles themselves is negligible compared to the overall volume of the gas.
Key Postulates of the Kinetic Molecular Theory:
- Particles are in constant, random motion: Gas particles are constantly moving in straight lines until they collide with each other or the walls of their container. This constant motion is responsible for the gas's pressure.
- Collisions are elastic: When gas particles collide, there is no net loss of kinetic energy. The total kinetic energy of the system remains constant.
- Negligible intermolecular forces: The attractive forces between gas particles are weak and insignificant compared to their kinetic energy. This allows the particles to move freely and independently.
- Average kinetic energy is proportional to temperature: The average kinetic energy of gas particles is directly proportional to the absolute temperature (Kelvin) of the gas. Higher temperatures mean faster-moving particles.
- Volume of particles is negligible: The volume occupied by the individual gas particles is insignificant compared to the total volume of the gas.
Why Gases Don't Have a Definite Shape: The Role of Particle Movement
The lack of a definite shape in gases is a direct consequence of the constant, random motion of their constituent particles. Unlike solids, where particles are tightly bound in a fixed arrangement, gas particles are free to move in all directions. They readily fill the entire available space within their container, adapting to the container's shape.
Imagine filling a balloon with air. The air molecules inside are not confined to a specific shape; instead, they spread out to occupy the entire volume of the balloon. If you change the shape of the balloon by squeezing it, the gas molecules will adjust their positions to conform to the new shape. This demonstrates the adaptability of gases and their lack of a fixed form.
Why Gases Don't Have a Definite Volume: The Role of Intermolecular Forces and Kinetic Energy
The absence of a definite volume in gases is linked to both the weak intermolecular forces and the high kinetic energy of their particles. Because the attractive forces between gas particles are negligible, they do not hold a fixed volume. The particles are constantly moving and colliding, expanding to fill any available space.
Consider the following scenarios:
- A gas in a closed container: The gas expands to fill the entire volume of the container. The gas molecules will distribute themselves uniformly throughout the container, regardless of its size or shape.
- A gas released into the atmosphere: The gas will expand indefinitely unless constrained by other forces such as gravity or other surrounding gases. The molecules will spread out until their concentration is sufficiently low.
The kinetic energy of the gas molecules plays a crucial role. The higher the temperature, the greater the kinetic energy, and the more the gas will expand. This is why gases are more easily compressed at lower temperatures – the lower kinetic energy allows for a smaller volume.
Factors Affecting Gas Behavior: Pressure, Temperature, and Volume
The behavior of gases is governed by several key factors:
- Pressure (P): Pressure is the force exerted by gas molecules per unit area on the walls of their container. It is directly related to the number of collisions between gas molecules and the container walls. Higher pressure indicates more frequent and forceful collisions.
- Volume (V): The volume of a gas is the space occupied by the gas molecules. As mentioned, gases do not have a definite volume, and it changes with pressure and temperature.
- Temperature (T): Temperature is a measure of the average kinetic energy of gas molecules. Higher temperatures mean faster-moving particles and thus higher pressure at a constant volume.
- Amount of gas (n): The number of moles of gas (n) is directly proportional to the pressure, at a constant volume and temperature. More gas molecules mean more collisions and higher pressure.
These factors are intricately linked, and their relationships are described by several gas laws:
- Boyle's Law: At constant temperature, the pressure of a gas is inversely proportional to its volume (P1V1 = P2V2).
- Charles's Law: At constant pressure, the volume of a gas is directly proportional to its absolute temperature (V1/T1 = V2/T2).
- Gay-Lussac's Law: At constant volume, the pressure of a gas is directly proportional to its absolute temperature (P1/T1 = P2/T2).
- Avogadro's Law: At constant temperature and pressure, the volume of a gas is directly proportional to the number of moles of gas (V1/n1 = V2/n2).
- Ideal Gas Law: Combines Boyle's, Charles's, and Avogadro's laws to give PV = nRT, where R is the ideal gas constant.
Deviations from Ideal Gas Behavior: Real Gases
The ideal gas law provides a good approximation of gas behavior under many conditions. However, real gases deviate from ideal behavior at high pressures and low temperatures. This is because:
- Intermolecular forces: At high pressures, gas molecules are closer together, and intermolecular forces become significant, affecting their movement and pressure.
- Volume of gas molecules: At high pressures and low temperatures, the volume occupied by the gas molecules themselves becomes a significant fraction of the total volume, contradicting the KMT assumption of negligible particle volume.
Understanding these deviations is crucial in many applications, particularly in chemical engineering and process design. Modified equations of state, such as the van der Waals equation, are used to account for these deviations and provide a more accurate description of real gas behavior.
Conclusion: The Dynamic Nature of Gases
In summary, gases do not possess a definite shape or volume because of the inherent nature of their constituent particles. The constant, random motion of these particles, coupled with weak intermolecular forces, allows them to readily conform to the shape and fill the entire volume of their container. The behavior of gases is governed by several key factors, primarily pressure, temperature, volume, and the amount of gas. While the ideal gas law provides a useful approximation, real gases deviate from this ideal behavior under certain conditions, necessitating more sophisticated models for accurate predictions. Understanding the properties of gases is fundamental to various scientific disciplines and practical applications, highlighting the dynamic and ever-changing nature of this state of matter. Further exploration into the complexities of gas behavior reveals a rich tapestry of scientific understanding, with ongoing research continuously refining our comprehension of these fascinating materials.
Latest Posts
Latest Posts
-
The Number Of Protons In An Atom Is That Elements
Apr 02, 2025
-
Is Magnesium Oxide Ionic Or Covalent
Apr 02, 2025
-
What Is The Subunit For Carbohydrates
Apr 02, 2025
-
X 2 X 1 0 Solve For X
Apr 02, 2025
-
Does Transcription Take Place In The Nucleus
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Does A Gas Have A Definite Shape And Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
