Does Ccl4 Have Dipole Dipole Forces
listenit
Mar 31, 2025 · 5 min read
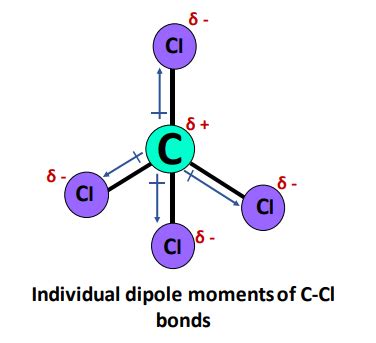
Table of Contents
Does CCl4 Have Dipole-Dipole Forces? Understanding Molecular Geometry and Intermolecular Forces
The question of whether carbon tetrachloride (CCl₄) exhibits dipole-dipole forces is a common one in chemistry, often used to test understanding of molecular geometry and intermolecular forces. The answer, however, isn't a simple yes or no. To fully understand why, we need to delve into the concepts of molecular polarity, bond polarity, and the different types of intermolecular forces.
Understanding Polarity: The Key to Intermolecular Forces
Before we address CCl₄ specifically, let's establish a foundational understanding of polarity. Polarity refers to the uneven distribution of electron density within a molecule or a bond. This uneven distribution creates a dipole, a separation of positive and negative charges. A molecule is considered polar if it possesses a net dipole moment, meaning the individual bond dipoles don't cancel each other out. Conversely, a nonpolar molecule has a net dipole moment of zero.
Bond Polarity: The Foundation of Molecular Polarity
Bond polarity arises from the difference in electronegativity between the atoms forming the bond. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. When two atoms with significantly different electronegativities bond, the electrons are drawn more towards the more electronegative atom, creating a polar bond. This results in a partial negative charge (δ-) on the more electronegative atom and a partial positive charge (δ+) on the less electronegative atom.
Molecular Geometry and Dipole Moment Cancellation
Even if a molecule contains polar bonds, the overall molecule may be nonpolar if the geometry of the molecule allows the individual bond dipoles to cancel each other out. This cancellation depends heavily on the molecule's symmetry. Symmetrical molecules often have nonpolar characteristics despite containing polar bonds.
Analyzing CCl₄: Geometry and Polarity
Now let's apply these concepts to carbon tetrachloride (CCl₄). CCl₄ has a central carbon atom bonded to four chlorine atoms. The arrangement of these atoms is tetrahedral, a highly symmetrical geometry.
The Role of Electronegativity in C-Cl Bonds
The electronegativity of chlorine is higher than that of carbon. Therefore, each C-Cl bond is polar, with the electrons being slightly more attracted to the chlorine atoms. This creates individual bond dipoles pointing from the carbon atom towards each chlorine atom.
Tetrahedral Symmetry and Dipole Moment Cancellation in CCl₄
Because of the perfect tetrahedral symmetry of CCl₄, these four individual bond dipoles are arranged in a way that they perfectly cancel each other out. The vector sum of the bond dipoles is zero. This means that despite the presence of polar C-Cl bonds, the molecule as a whole has a zero dipole moment.
Intermolecular Forces in CCl₄: London Dispersion Forces Dominate
Since CCl₄ is a nonpolar molecule, it does not exhibit dipole-dipole forces. Instead, the primary intermolecular forces present in CCl₄ are London Dispersion Forces (LDFs), also known as van der Waals forces.
Understanding London Dispersion Forces (LDFs)
LDFs are the weakest type of intermolecular force, but they are present in all molecules, both polar and nonpolar. They arise from temporary, instantaneous fluctuations in electron distribution around atoms and molecules. These fluctuations create temporary dipoles, which can induce dipoles in neighboring molecules, resulting in weak attractive forces.
The Strength of LDFs in CCl₄
In the case of CCl₄, the large size of the chlorine atoms and the molecule's overall size contribute to relatively strong LDFs. The larger the molecule and the greater the number of electrons, the stronger the LDFs become. This explains why CCl₄, despite being nonpolar, has a relatively high boiling point compared to some smaller nonpolar molecules.
Comparing CCl₄ with Polar Molecules: A Contrast in Intermolecular Forces
To further highlight the absence of dipole-dipole forces in CCl₄, let's compare it to a polar molecule like chloroform (CHCl₃).
Chloroform (CHCl₃): A Polar Molecule
Chloroform has a similar tetrahedral geometry to CCl₄, but the replacement of one chlorine atom with a hydrogen atom breaks the symmetry. This asymmetry prevents the bond dipoles from completely canceling each other out, resulting in a net dipole moment. Therefore, chloroform is a polar molecule.
Intermolecular Forces in Chloroform: Dipole-Dipole Forces Present
Because chloroform is polar, it exhibits dipole-dipole forces in addition to LDFs. These dipole-dipole forces are stronger than LDFs, contributing to chloroform's higher boiling point compared to CCl₄.
Practical Implications and Applications
Understanding the intermolecular forces present in CCl₄ has practical implications. The relatively strong LDFs contribute to its properties as a solvent for nonpolar substances. However, its nonpolar nature limits its ability to dissolve polar substances.
The fact that CCl₄ is nonpolar and relies solely on LDFs also impacts its environmental behaviour. While once widely used as a solvent and refrigerant, its contribution to ozone depletion led to its phase-out. The lack of strong intermolecular forces with water molecules also hinders its solubility and facilitates its migration through the environment.
Conclusion: CCl₄ and the Importance of Molecular Geometry
In conclusion, while CCl₄ possesses polar C-Cl bonds, its tetrahedral symmetry leads to a zero dipole moment, making it a nonpolar molecule. Therefore, CCl₄ does not exhibit dipole-dipole forces. Its intermolecular forces are dominated by London Dispersion Forces. This understanding is crucial for comprehending its physical and chemical properties, as well as its environmental impact. The example of CCl₄ underscores the vital role of molecular geometry in determining a molecule's overall polarity and consequently, its intermolecular interactions. This knowledge is fundamental to many areas of chemistry, from predicting boiling points and solubility to understanding reaction mechanisms and environmental behavior. The careful consideration of both bond polarity and molecular symmetry is essential for accurately predicting the types of intermolecular forces present in any molecule.
Latest Posts
Latest Posts
-
What Is The Square Root Of 122
Apr 02, 2025
-
The Unit Of Energy In Si Units Is
Apr 02, 2025
-
How To Find The Mean Of A Probability Distribution
Apr 02, 2025
-
How Do Producers Get Their Energy
Apr 02, 2025
-
The Number Of Protons In An Atom Is That Elements
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Does Ccl4 Have Dipole Dipole Forces . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
