Sublimation Is Physical Or Chemical Change
listenit
Apr 06, 2025 · 5 min read
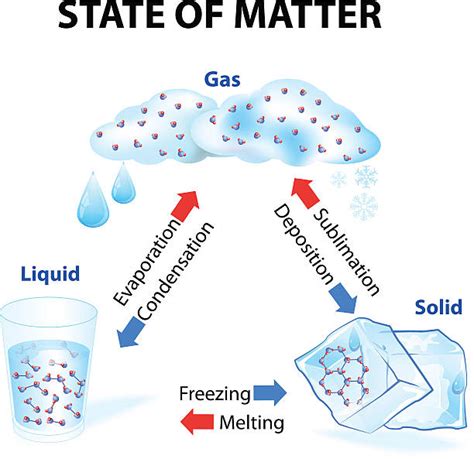
Table of Contents
Sublimation: A Physical Change, Not a Chemical One
The question of whether sublimation is a physical or chemical change is a common one, especially in introductory chemistry courses. Understanding the difference between physical and chemical changes is crucial for grasping fundamental concepts in science. This comprehensive article will delve into the nature of sublimation, solidifying the understanding that it is indeed a physical change, exploring the underlying principles, providing illustrative examples, and addressing potential misconceptions.
Defining Physical and Chemical Changes
Before we delve into sublimation, let's establish clear definitions of physical and chemical changes.
Physical Changes
A physical change alters the form or appearance of a substance but doesn't change its chemical composition. The substance remains the same; only its physical properties (like shape, size, or state) are modified. These changes are often reversible. Examples include:
- Melting: Ice turning into water.
- Boiling: Water turning into steam.
- Freezing: Water turning into ice.
- Dissolving: Salt dissolving in water.
- Crushing: Crushing a rock into smaller pieces.
These changes affect the physical state of the matter but not its molecular structure. The molecules themselves remain unchanged.
Chemical Changes
A chemical change, also known as a chemical reaction, involves the transformation of one or more substances into entirely new substances with different chemical properties. These changes are often irreversible and involve the breaking and forming of chemical bonds. Examples include:
- Burning: Wood burning in a fire.
- Rusting: Iron reacting with oxygen to form rust.
- Digestion: The breakdown of food in the body.
- Cooking: Chemical changes occur when food is cooked.
Understanding Sublimation: A Deep Dive
Sublimation is the transition of a substance directly from the solid to the gaseous phase without passing through the intermediate liquid phase. This process occurs when the substance's solid phase has a high enough vapor pressure at a given temperature. In simpler terms, some solids can directly turn into gas, skipping the liquid stage.
Key Characteristics of Sublimation:
- Direct Solid-to-Gas Transition: This is the defining characteristic of sublimation. There is no melting stage involved.
- Endothermic Process: Sublimation requires energy input (heat) to overcome the intermolecular forces holding the solid together.
- Reversible Process (Usually): Under the right conditions, the reverse process, deposition (gas to solid), can occur.
- Dependent on Vapor Pressure and Temperature: The ability of a solid to sublimate depends on its vapor pressure and the surrounding temperature and pressure.
Why Sublimation is a Physical Change
Sublimation is classified as a physical change because it doesn't involve a change in the chemical composition of the substance. The molecules of the substance remain the same throughout the process. Only the spacing and arrangement of these molecules change as the substance transitions from the solid to the gaseous state.
Think of it this way: when dry ice (solid carbon dioxide) sublimates, it turns into carbon dioxide gas. The chemical formula remains CO2; only the physical state changes from solid to gas. No new chemical substance is formed.
Evidence Supporting Sublimation as a Physical Change:
- Reversibility: Sublimation is often a reversible process. Under specific conditions, the gaseous form can return to its solid state through deposition, demonstrating that the chemical identity remains intact.
- No New Substances Formed: The chemical composition of the substance remains unchanged throughout the sublimation process. This is the most critical factor in classifying it as a physical change.
- Changes in Physical Properties Only: Sublimation only involves changes in physical properties such as state, density, and volume. Chemical properties like reactivity and flammability remain unchanged.
Examples of Sublimation
Several substances readily undergo sublimation. Here are some well-known examples:
- Dry Ice (Solid Carbon Dioxide): This is the most common example. Dry ice sublimates at room temperature and atmospheric pressure, producing a visible cloud of carbon dioxide gas.
- Naphthalene (Mothballs): These slowly sublimate over time, releasing their characteristic odor. This sublimation is what makes them effective in repelling moths.
- Iodine: Solid iodine crystals can directly sublimate into a purple vapor when heated gently.
- Camphor: Like naphthalene, camphor also exhibits sublimation, releasing a distinctive aroma.
- Arsenic Trioxide: This substance sublimes at relatively low temperatures.
Factors Affecting Sublimation
Several factors influence the rate and extent of sublimation:
- Temperature: Higher temperatures generally accelerate sublimation.
- Pressure: Lower pressures favor sublimation. At higher pressures, the substance is more likely to melt before sublimating.
- Surface Area: A larger surface area of the solid exposes more molecules to the environment, increasing the rate of sublimation.
- Vapor Pressure: Substances with a high vapor pressure at a given temperature are more likely to sublimate.
Addressing Common Misconceptions
Some might confuse sublimation with evaporation, which is also a phase change. However, there's a crucial difference:
- Evaporation: This is the transition of a liquid to a gas. Sublimation skips the liquid phase entirely.
Another potential misconception is that sublimation is always fast. While some substances sublimate rapidly, others do so very slowly over time. The rate depends on the factors mentioned earlier.
Sublimation in Everyday Life and Industrial Applications
Sublimation finds numerous applications in everyday life and industrial processes:
- Freeze-drying: This food preservation technique utilizes sublimation to remove water from frozen food, preserving its quality and flavor.
- Purification of substances: Sublimation can be used to purify substances by separating them from impurities that don't sublimate at the same conditions.
- Printing: Sublimation printing utilizes sublimation to transfer dye onto various substrates like textiles and ceramics.
- Metal Deposition: Sublimation is employed in the deposition of thin films of metals in various industries.
Conclusion
In conclusion, sublimation is definitively a physical change, not a chemical one. The process involves only a change in the physical state of a substance, with no alteration in its chemical composition. Understanding this distinction is key to grasping the fundamentals of chemistry and appreciating the diverse ways in which matter can transform. The applications of sublimation span various fields, highlighting its significance in both everyday life and industrial processes. This comprehensive exploration has clarified the nature of sublimation, debunking common misconceptions and emphasizing its importance as a fundamental physical process.
Latest Posts
Latest Posts
-
Is An Isosceles Trapezoid A Parallelogram
Apr 07, 2025
-
What Is The Molar Mass Of Pbso4
Apr 07, 2025
-
Common Factors Of 8 And 36
Apr 07, 2025
-
How Do You Factor X 3 125
Apr 07, 2025
-
What Is The Electron Configuration Of V
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Sublimation Is Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.