What Is The Molar Mass Of Pbso4
listenit
Apr 07, 2025 · 5 min read
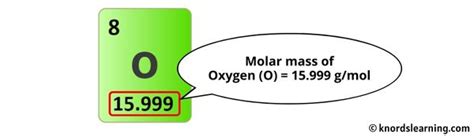
Table of Contents
What is the Molar Mass of PbSO₄? A Comprehensive Guide
Lead(II) sulfate (PbSO₄) is a white, crystalline, inorganic compound with a variety of applications, from lead-acid batteries to pigments. Understanding its molar mass is crucial in various chemical calculations and analyses. This article will delve deep into determining the molar mass of PbSO₄, explaining the process step-by-step and providing valuable context for its importance.
Understanding Molar Mass
Before calculating the molar mass of PbSO₄, it's essential to grasp the fundamental concept of molar mass. Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of entities, whether atoms, molecules, ions, or formula units. The molar mass is numerically equivalent to the atomic or molecular weight but expressed in grams per mole (g/mol).
Importance of Molar Mass
Knowing the molar mass is critical for various applications in chemistry, including:
- Stoichiometry: Calculating the quantities of reactants and products in chemical reactions.
- Solution Chemistry: Determining the concentration of solutions (e.g., molarity).
- Titrations: Analyzing the concentration of unknown solutions through titration techniques.
- Gravimetric Analysis: Determining the amount of a substance in a sample using mass measurements.
- Spectroscopy: Relating the measured signal to the amount of substance present.
Calculating the Molar Mass of PbSO₄
To calculate the molar mass of PbSO₄, we need to consider the atomic masses of its constituent elements: lead (Pb), sulfur (S), and oxygen (O). These atomic masses are typically found on the periodic table of elements.
Step 1: Identify the Elements and their Atomic Masses
- Lead (Pb): The atomic mass of lead is approximately 207.2 g/mol. Note that this value might slightly vary depending on the isotopic abundance used in the periodic table. Slight variations in reported atomic masses are common and acceptable for most practical purposes.
- Sulfur (S): The atomic mass of sulfur is approximately 32.1 g/mol.
- Oxygen (O): The atomic mass of oxygen is approximately 16.0 g/mol.
Step 2: Determine the Number of Atoms of Each Element
The chemical formula PbSO₄ indicates that one formula unit of lead(II) sulfate contains:
- 1 lead (Pb) atom
- 1 sulfur (S) atom
- 4 oxygen (O) atoms
Step 3: Calculate the Total Molar Mass
To determine the molar mass of PbSO₄, we sum the atomic masses of each element, taking into account the number of atoms of each element present in the formula unit:
Molar Mass (PbSO₄) = (1 × Atomic Mass of Pb) + (1 × Atomic Mass of S) + (4 × Atomic Mass of O)
Molar Mass (PbSO₄) = (1 × 207.2 g/mol) + (1 × 32.1 g/mol) + (4 × 16.0 g/mol)
Molar Mass (PbSO₄) = 207.2 g/mol + 32.1 g/mol + 64.0 g/mol
Molar Mass (PbSO₄) = 303.3 g/mol
Therefore, the molar mass of PbSO₄ is approximately 303.3 grams per mole.
Practical Applications and Examples
The molar mass of PbSO₄ is crucial in various practical applications. Let's consider a few examples:
Example 1: Stoichiometry Calculation
Let's say we want to determine the mass of PbSO₄ produced from a reaction involving a certain amount of lead. Knowing the molar mass allows us to convert moles of PbSO₄ to grams and vice-versa.
For example, if a reaction produces 0.5 moles of PbSO₄, we can calculate the mass:
Mass (PbSO₄) = Moles (PbSO₄) × Molar Mass (PbSO₄)
Mass (PbSO₄) = 0.5 mol × 303.3 g/mol = 151.65 g
Example 2: Solution Preparation
If we want to prepare a 1 M solution of PbSO₄, we need to know its molar mass to accurately weigh out the required amount. One liter of a 1 M solution contains 1 mole of solute. Therefore, we would weigh out 303.3 g of PbSO₄ and dissolve it in enough solvent to make a 1-liter solution.
Example 3: Gravimetric Analysis
In gravimetric analysis, we might precipitate PbSO₄ from a solution and weigh the resulting precipitate. Knowing the molar mass allows us to calculate the amount of lead originally present in the solution based on the mass of PbSO₄ obtained.
Factors Affecting Molar Mass Values
While the calculated molar mass of 303.3 g/mol is a good approximation, minor variations can occur due to several factors:
- Isotopic Abundance: The atomic masses listed in periodic tables are usually weighted averages based on the isotopic abundance of each element. Slight variations in isotopic abundance can lead to minor differences in the calculated molar mass.
- Measurement Precision: The precision of the instruments used to determine atomic masses influences the accuracy of the molar mass calculation.
- Rounding Errors: Rounding off atomic masses during the calculation can introduce minor errors.
However, these variations are typically insignificant for most practical applications. The value of 303.3 g/mol provides a sufficiently accurate approximation for everyday chemical calculations.
Conclusion
The molar mass of PbSO₄, approximately 303.3 g/mol, is a fundamental parameter in various chemical calculations. Understanding how to calculate this value and its implications in stoichiometry, solution preparation, and analytical techniques is crucial for any chemist or anyone working with lead(II) sulfate. This detailed explanation provides a solid foundation for understanding this essential concept and its practical applications in the realm of chemistry. The accurate determination of molar mass is vital for precise and reliable results in chemical analysis and experimental work. Remember to always refer to a reliable source for the most up-to-date atomic mass values for the most accurate calculations.
Latest Posts
Latest Posts
-
Electron Dot Structure Of Hydrogen Chloride
Apr 07, 2025
-
How Many Electrons Are In Mg 2
Apr 07, 2025
-
Whats 3 5 As A Decimal
Apr 07, 2025
-
In Glycolysis What Is Oxidized And What Is Reduced
Apr 07, 2025
-
A Word That Describes Or Modifies A Noun
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Pbso4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.