What Is The Electron Configuration Of V
listenit
Apr 07, 2025 · 5 min read
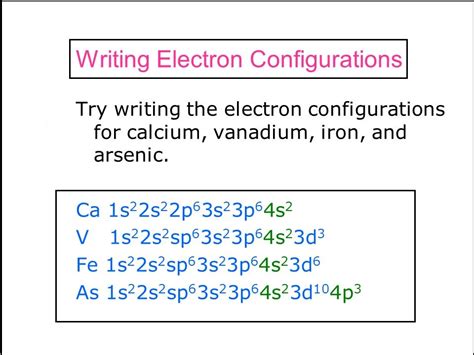
Table of Contents
What is the Electron Configuration of Vanadium (V)? A Deep Dive into Atomic Structure
Vanadium, a transition metal with the symbol V and atomic number 23, holds a fascinating place in the periodic table. Its unique electronic structure is responsible for its diverse chemical properties and applications in various fields, from metallurgy to biology. Understanding the electron configuration of vanadium is key to grasping its behavior and importance. This article will provide a comprehensive exploration of vanadium's electron configuration, explaining the underlying principles and its implications.
Understanding Electron Configurations
Before diving into the specifics of vanadium, let's establish a foundational understanding of electron configuration. The electron configuration of an atom describes how electrons are distributed among the various energy levels and sublevels within the atom. These electrons occupy orbitals, regions of space where there's a high probability of finding an electron. This arrangement is governed by several fundamental principles:
The Aufbau Principle
The Aufbau principle dictates that electrons fill the lowest energy levels first. This means electrons fill orbitals starting with the lowest energy level (1s) and progressively moving to higher energy levels (2s, 2p, 3s, 3p, and so on).
Hund's Rule
Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to a more stable configuration.
The Pauli Exclusion Principle
The Pauli exclusion principle asserts that no two electrons in an atom can have the same set of four quantum numbers. This means that each orbital can hold a maximum of two electrons, each with opposite spins (designated as +1/2 and -1/2).
Determining the Electron Configuration of Vanadium (V)
Vanadium has an atomic number of 23, meaning it has 23 protons and, in its neutral state, 23 electrons. Following the Aufbau principle, Hund's rule, and the Pauli exclusion principle, we can systematically determine its electron configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d³
Let's break this down:
- 1s²: Two electrons fill the 1s orbital (lowest energy level).
- 2s²: Two electrons fill the 2s orbital.
- 2p⁶: Six electrons fill the three 2p orbitals (px, py, pz).
- 3s²: Two electrons fill the 3s orbital.
- 3p⁶: Six electrons fill the three 3p orbitals.
- 4s²: Two electrons fill the 4s orbital. Note: While the 4s orbital is generally filled before the 3d orbital, exceptions exist, and we'll delve into that further.
- 3d³: Three electrons partially fill the five 3d orbitals. This partial filling is characteristic of transition metals and contributes to vanadium's unique properties.
Therefore, the full electron configuration of vanadium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d³.
The Subtleties of Transition Metal Electron Configurations: The Case of Vanadium
The filling of orbitals in transition metals, like vanadium, can be slightly more complex than in main group elements. The energy difference between the (n-1)d and ns orbitals is relatively small. This means that in some cases, electrons can be promoted from the (n-1)d subshell to the ns subshell, or even to a higher energy level, resulting in what's known as an "irregular" or "exceptional" configuration. Although the predicted configuration based on the Aufbau principle is 4s²3d³, there's a degree of orbital mixing involved in the actual electronic structure.
However, for most practical purposes and general chemistry discussions, the 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d³ configuration provides a good representation of the electronic structure of vanadium.
Noble Gas Configuration and Condensed Notation
A simplified way to represent electron configuration is to use noble gas notation. This involves replacing the core electrons with the symbol of the noble gas that precedes the element in the periodic table. In vanadium's case, the noble gas preceding it is Argon (Ar), with an electron configuration of 1s² 2s² 2p⁶ 3s² 3p⁶. Therefore, the condensed electron configuration of vanadium is:
[Ar] 4s² 3d³
This notation is concise and emphasizes the valence electrons (those in the outermost shell) that are primarily responsible for chemical bonding and reactivity.
The Importance of Vanadium's Electron Configuration
Vanadium's specific electron configuration is directly linked to its properties and applications:
-
Variable Oxidation States: The presence of partially filled 3d orbitals allows vanadium to exhibit multiple oxidation states (+2, +3, +4, and +5), making it versatile in chemical reactions. This ability to readily lose or gain electrons is a key feature of transition metals.
-
Catalysis: Vanadium compounds are effective catalysts in various chemical processes. The ability of vanadium to easily change its oxidation state is crucial for its catalytic activity. This is utilized in processes like the production of sulfuric acid and the oxidation of hydrocarbons.
-
Alloy Formation: Vanadium forms strong alloys with other metals, enhancing their strength and durability. These alloys find applications in high-performance steels and aerospace components. The d-electrons contribute to the metallic bonding characteristics.
-
Biological Roles: Vanadium plays a role in some biological systems, although its function is not fully understood. Some vanadium compounds have shown potential antimicrobial and antitumor activity. The electronic structure influences its interaction with biological molecules.
-
Magnetic Properties: The unpaired electrons in the 3d orbitals contribute to vanadium's paramagnetic properties, meaning it's weakly attracted to magnetic fields.
Further Exploration of Vanadium Chemistry
The electronic configuration of vanadium provides a foundation for understanding its rich and diverse chemistry. Further studies can delve into:
-
Vanadium Complexes: The interaction of vanadium ions with ligands (molecules or ions that bond to a central metal ion) forms various complexes with unique structures and properties. This area is extensively researched in coordination chemistry.
-
Spectroscopy of Vanadium Compounds: Techniques like UV-Vis spectroscopy and electron paramagnetic resonance (EPR) spectroscopy are used to study the electronic structure and properties of vanadium compounds.
-
Vanadium in Materials Science: Ongoing research explores vanadium's role in novel materials, such as superconductors and energy storage systems. The ability to fine-tune the electronic properties through alloying or doping opens up numerous possibilities.
Conclusion
The electron configuration of vanadium, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d³ ([Ar] 4s² 3d³), is not merely a theoretical construct; it's a key to understanding the atom's behavior and the element's remarkable properties. This configuration underpins vanadium's diverse oxidation states, catalytic activity, alloying behavior, and potential biological roles. Further research continues to uncover the full potential of this fascinating transition metal and its applications across various scientific and technological fields. Understanding its electronic structure is crucial for advancing our knowledge and utilizing vanadium's unique characteristics effectively.
Latest Posts
Latest Posts
-
Whats 3 5 As A Decimal
Apr 07, 2025
-
In Glycolysis What Is Oxidized And What Is Reduced
Apr 07, 2025
-
A Word That Describes Or Modifies A Noun
Apr 07, 2025
-
What Stores Food And Water In A Cell
Apr 07, 2025
-
Write 9 10 As A Decimal Number
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of V . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.