Is Water Boiling Physical Or Chemical Change
listenit
Mar 15, 2025 · 6 min read
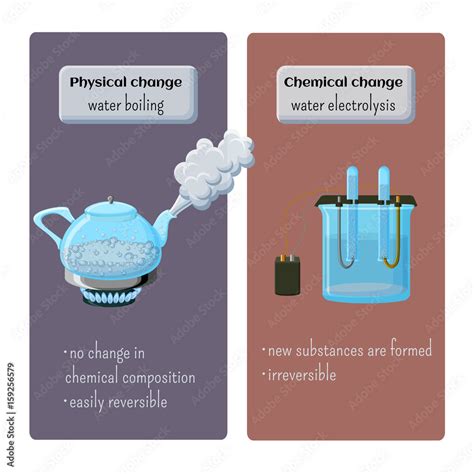
Table of Contents
Is Boiling Water a Physical or Chemical Change? A Deep Dive
The seemingly simple act of boiling water sparks a fundamental question in chemistry: is it a physical change or a chemical change? While the answer might seem obvious at first glance, a closer examination reveals a more nuanced understanding of the processes involved and the critical distinctions between physical and chemical transformations. This comprehensive exploration will delve into the intricacies of boiling water, examining the evidence supporting its classification as a physical change, while also addressing common misconceptions and related concepts.
Understanding Physical and Chemical Changes
Before we delve into the specifics of boiling water, let's establish a clear understanding of the defining characteristics of physical and chemical changes.
Physical Changes
Physical changes alter the form or appearance of a substance without changing its chemical composition. These changes are often reversible. Key indicators of a physical change include:
- Change in state: Melting, freezing, boiling, condensing, and sublimating are all examples of physical changes where the state of matter alters (solid, liquid, gas) but the chemical identity remains the same.
- Change in shape or size: Cutting, bending, or crushing a substance alters its physical form without changing its chemical makeup.
- Separation of mixtures: Processes like filtration or distillation separate components of a mixture without altering the chemical nature of each component. These are physical changes.
Chemical Changes
Chemical changes, also known as chemical reactions, involve the rearrangement of atoms and molecules, resulting in the formation of new substances with different properties. These changes are often irreversible. Key indicators of a chemical change include:
- Formation of a gas: The production of bubbles or fumes often signifies a chemical reaction.
- Formation of a precipitate: The appearance of a solid from a solution indicates a chemical reaction.
- Color change: A significant and unexpected color change can be a sign of a chemical reaction.
- Temperature change: A significant temperature increase (exothermic reaction) or decrease (endothermic reaction) often accompanies a chemical reaction.
- Irreversibility: Chemical changes are often difficult or impossible to reverse without further chemical reactions.
The Case for Boiling Water as a Physical Change
Boiling water is unequivocally a physical change. Let's examine the evidence:
1. No New Substance is Formed
When water boils, it transitions from its liquid state to its gaseous state (water vapor or steam). The chemical composition remains unchanged; it's still H₂O. The molecules are simply moving faster and further apart due to the increased thermal energy. No new chemical bonds are formed, and no existing ones are broken. This lack of a change in chemical composition is the hallmark of a physical change.
2. The Change is Reversible
The process of boiling water is easily reversible. By cooling the steam, it condenses back into liquid water. This cycle of evaporation and condensation demonstrates the reversible nature of the boiling process, solidifying its classification as a physical change. The water molecules remain intact throughout the entire process.
3. Only Energy Input Alters the State
Boiling water requires an input of energy (heat). This energy increases the kinetic energy of the water molecules, overcoming the intermolecular forces holding them together in the liquid phase. This increased kinetic energy allows the molecules to escape the liquid phase and enter the gaseous phase. However, the chemical structure of the water molecule itself remains unaffected. The energy simply changes the state of matter.
4. No Chemical Indicators are Present
None of the indicators of a chemical change (gas formation, precipitate formation, color change, significant temperature change beyond simple heating, irreversibility) are present during the boiling of water. The change in temperature is directly related to the energy input and doesn't represent a chemical reaction.
Addressing Common Misconceptions
Despite the clear evidence, some misconceptions surrounding the boiling of water persist. Let's address these common misunderstandings:
Misconception 1: Bubbles indicate a chemical reaction.
Clarification: The bubbles observed during boiling are not the result of a chemical reaction. They are simply water vapor (steam) forming and escaping the liquid phase due to the increased kinetic energy of the water molecules. These bubbles contain the same H₂O molecules as the liquid water.
Misconception 2: The change in temperature is a chemical indicator.
Clarification: The increase in temperature during boiling is a result of adding thermal energy, not a chemical reaction. The temperature increase facilitates the phase change from liquid to gas, but it doesn't signify the formation of a new substance.
Misconception 3: Decomposition of water occurs.
Clarification: While water can be decomposed through electrolysis (a chemical process using electricity), simple boiling does not decompose water. Electrolysis breaks down water molecules into hydrogen and oxygen gas, creating entirely new substances. Boiling merely changes the state, not the chemical composition.
Electrolysis vs. Boiling: A Crucial Distinction
It's essential to differentiate between boiling water and the electrolysis of water. Electrolysis is a chemical change because it breaks down water (H₂O) into its constituent elements, hydrogen (H₂) and oxygen (O₂). This involves the breaking and forming of chemical bonds, resulting in entirely new substances with different properties. Boiling, on the other hand, only changes the physical state of the water without altering its chemical composition.
The Role of Impurities
While pure water undergoes a straightforward physical change when boiled, the presence of impurities can complicate matters slightly. Impurities can affect the boiling point of water and might even lead to minor chemical reactions at very high temperatures. However, these reactions are typically independent of the boiling process itself. The boiling of the water remains a physical change even with impurities present; the chemical reactions of the impurities are separate events.
For example, dissolved minerals in hard water might precipitate out as the water evaporates, but this precipitation is a separate chemical process, not inherent to the boiling of the water. The majority of the water undergoes a simple phase change.
Conclusion: Boiling Water is a Physical Change
In conclusion, the overwhelming evidence supports the classification of boiling water as a physical change. The process involves a change in state from liquid to gas, but the chemical composition (H₂O) remains unchanged. The change is reversible, no new substances are formed, and there are no indicators of a chemical reaction. While impurities can introduce complexities, the fundamental process of water transitioning from liquid to gas remains a physical transformation. Understanding this distinction is crucial for grasping the fundamentals of chemistry and differentiating between physical and chemical processes. The seemingly simple act of boiling water provides a valuable example of a fundamental physical change and serves as an excellent illustration to differentiate between physical and chemical transformations in the realm of chemistry.
Latest Posts
Latest Posts
-
P 10 P 7 8 9
Mar 15, 2025
-
How To Get Rid Of A Fraction
Mar 15, 2025
-
Number Of Valence Electrons Of Sodium
Mar 15, 2025
-
How Many Sigma And Pi Bonds In A Triple Bond
Mar 15, 2025
-
Why Does Active Transport Need Energy
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is Water Boiling Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
