Is Color A Physical Or Chemical Change
listenit
Mar 25, 2025 · 6 min read
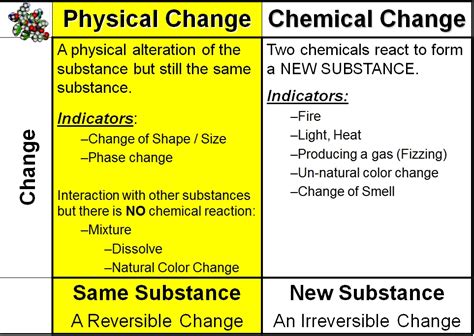
Table of Contents
Is Color a Physical or Chemical Change? A Deep Dive into the Science of Color
The question of whether a change in color signifies a physical or chemical alteration is a surprisingly complex one. While it's tempting to assume that a color change always indicates a chemical reaction, the reality is far more nuanced. The truth lies in understanding the underlying causes of color and the nature of the changes involved. This article will delve deep into the science of color, exploring the different ways color can change and definitively determining when a color change represents a physical versus a chemical transformation.
Understanding Color: The Foundation of Our Perception
Before we can tackle the physical versus chemical debate, we need a firm grasp on what color actually is. Color isn't an inherent property of an object; rather, it's our perception of how an object interacts with light. Light, which is electromagnetic radiation, exists as a spectrum of wavelengths. When light strikes an object, the object can absorb, reflect, or transmit different wavelengths. The wavelengths that are reflected are the ones we perceive as color.
Absorption and Reflection: The Key Players
-
Absorption: When an object absorbs certain wavelengths of light, those wavelengths are not reflected to our eyes. For example, a red apple absorbs most wavelengths except for red, which it reflects.
-
Reflection: The wavelengths of light that are not absorbed are reflected. These reflected wavelengths determine the color we see. A perfectly white object reflects all wavelengths equally, while a perfectly black object absorbs all wavelengths.
-
Transmission: Some materials, like glass, transmit light, allowing wavelengths to pass through. The color we see depends on which wavelengths are transmitted and which are absorbed.
Physical Changes and Color: No New Substances Formed
A physical change alters the form or appearance of a substance without changing its chemical composition. Think of cutting a piece of paper, melting ice, or dissolving sugar in water. These changes are reversible, and the chemical identity of the substance remains intact. In the context of color, physical changes can influence the way light interacts with a substance, leading to a change in its perceived color.
Examples of Physical Color Changes:
-
Dissolving: Dissolving a colored substance in a solvent might alter the intensity or appearance of the color. For instance, dissolving blue dye in water will create a blue solution. However, the dye molecules themselves haven't undergone a chemical change; they're simply dispersed in the water. This is a physical change.
-
Mixing: Combining different colored substances can result in a new color, but this is a physical mixture, not a chemical reaction. For instance, mixing blue and yellow paints creates green. The individual pigments remain chemically unchanged.
-
Changes in State: The state of a substance (solid, liquid, gas) can influence its interaction with light. For example, solid iodine is dark purple-black, while gaseous iodine is violet. This is a physical change; the iodine molecules are still iodine molecules.
-
Grinding or Crushing: Physically altering the size and shape of particles can affect how light scatters, influencing color perception. For example, finely ground pigments appear more intense than their coarser counterparts due to increased surface area interaction with light.
-
Temperature Changes: In some cases, temperature can induce changes in the crystal structure of a substance, affecting light scattering and hence, color. The chemical composition, however, remains unchanged.
Chemical Changes and Color: New Substances Are Formed
A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules to form new substances. These changes are often irreversible, and the properties of the resulting substances differ significantly from the original reactants. Chemical reactions frequently lead to color changes due to the formation of new compounds with different light-absorbing and reflecting properties.
Examples of Chemical Color Changes:
-
Rusting: Iron reacting with oxygen and water forms iron oxide (rust), a reddish-brown substance distinctly different from the original iron. This is a chemical change accompanied by a dramatic color change.
-
Burning: Combustion involves a rapid chemical reaction with oxygen, typically producing heat and light. The color change is a consequence of the formation of new compounds, such as carbon dioxide and water, from the original fuel.
-
Reactions with Acids and Bases: Many chemical reactions involving acids and bases produce characteristic color changes. For example, adding an acid to a base can lead to a neutralization reaction and a change in color, often indicated by the use of an acid-base indicator.
-
Enzyme Reactions: Enzymes, biological catalysts, can catalyze reactions that lead to color changes. Many enzymatic assays rely on colorimetric detection, where the product of the enzymatic reaction has a different color than the reactants.
-
Photochromism: Certain substances exhibit photochromism, changing color in response to light exposure. This is a chemical change as the molecular structure alters reversibly under the influence of light.
Distinguishing Physical from Chemical Color Changes: Key Considerations
Determining whether a color change is physical or chemical often requires a careful analysis of the process involved. Here are some crucial factors to consider:
-
Reversibility: Physical changes are often reversible, while chemical changes are typically irreversible. However, this isn't always a foolproof indicator, as some chemical reactions can be reversed under specific conditions.
-
Formation of new substances: The formation of entirely new chemical compounds is a definitive sign of a chemical change. This can often be confirmed through techniques like spectroscopy or chromatography.
-
Energy changes: Chemical reactions often involve significant energy changes, such as the release or absorption of heat or light. While physical changes might involve energy changes, they are typically less dramatic.
-
Observation of other changes: Look for other indicators of a chemical change, such as the formation of a precipitate, the release of a gas, or a change in odor or temperature.
Beyond the Basics: Advanced Concepts in Color Change
The relationship between color and chemical/physical changes is more multifaceted than initially appears. Several advanced concepts further complicate the classification:
-
Structural Color: Certain colors arise not from chemical pigments but from the physical structure of a material. This is often seen in iridescent materials, where the color changes depending on the angle of observation. These color changes are fundamentally physical, caused by interference and diffraction of light.
-
Chromophores and Auxochromes: In organic chemistry, chromophores are specific groups of atoms that absorb light in the visible spectrum, causing color. Auxochromes are groups that modify the absorption properties of chromophores, resulting in a shift in color. Changes in these groups can lead to color shifts that may be considered either physical or chemical, depending on the nature of the modification.
-
Quantum Dots: These are semiconductor nanocrystals whose color is size-dependent. Changing the size of the quantum dots through physical means, like altering the synthesis conditions, leads to a color change. While the chemical composition remains largely the same, the size-dependent quantum effects lead to a color change. This blurs the lines further between physical and chemical changes related to color.
Conclusion: A Nuance-Rich Phenomenon
The connection between color and chemical or physical changes is far from straightforward. While a color change often suggests a chemical reaction, it can equally result from purely physical alterations. The key lies in analyzing the underlying mechanisms of the color change, considering factors like reversibility, the formation of new substances, energy changes, and any other accompanying changes. Understanding the fundamental nature of light and its interaction with matter is paramount in accurately classifying these color transformations. By carefully considering these aspects, we can develop a deeper appreciation for the complex relationship between color and the physical and chemical world around us. This nuanced understanding is crucial for fields like materials science, chemistry, and even art, where the manipulation of color is critical.
Latest Posts
Latest Posts
-
Graph The Linear Equation X 4
Mar 27, 2025
-
What Are Two Functional Groups Found In Amino Acids
Mar 27, 2025
-
2 3 Divided By 3 4
Mar 27, 2025
-
What Subatomic Particles Make Up An Atom
Mar 27, 2025
-
How To Find Ph Of Buffer Solution
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Is Color A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
