How To Find Ph Of Buffer Solution
listenit
Mar 27, 2025 · 6 min read
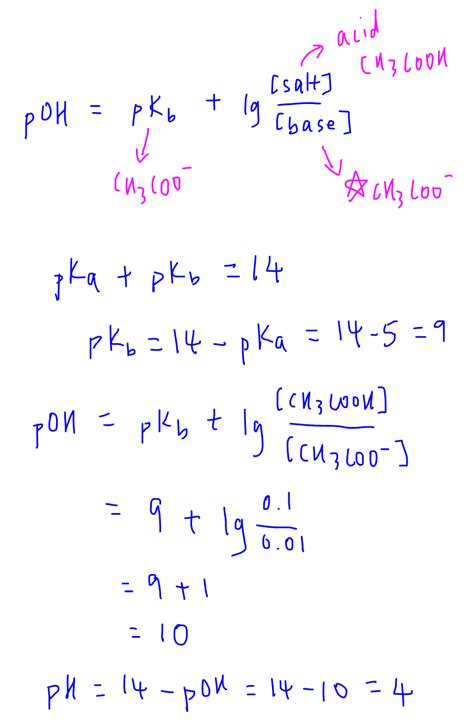
Table of Contents
How to Find the pH of a Buffer Solution: A Comprehensive Guide
Determining the pH of a buffer solution is crucial in numerous scientific and industrial applications. Buffers maintain a relatively stable pH even when small amounts of acid or base are added, making them essential in processes requiring precise pH control. This comprehensive guide will explore various methods for calculating and measuring the pH of buffer solutions, covering theoretical concepts and practical techniques.
Understanding Buffer Solutions and Their Importance
Before delving into the methods for determining pH, let's solidify our understanding of buffer solutions themselves. A buffer solution, also known as a pH buffer, is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or a weak base and its conjugate acid. This combination resists changes in pH upon the addition of small amounts of strong acid or base. This property is vital in numerous contexts:
-
Biological Systems: Maintaining a stable pH is essential for the proper functioning of biological systems. Blood, for instance, relies on a bicarbonate buffer system to maintain its pH around 7.4. Enzyme activity and cellular processes are highly sensitive to pH changes, highlighting the importance of buffers in biological research and applications.
-
Chemical Reactions: Many chemical reactions require specific pH conditions for optimal performance. Buffers ensure these conditions remain constant, allowing for consistent and predictable reaction outcomes.
-
Industrial Processes: Numerous industrial processes, such as food preservation, pharmaceuticals manufacturing, and water treatment, use buffers to control pH. Maintaining a controlled pH is crucial for the quality, stability, and effectiveness of the final product.
Calculating the pH of a Buffer Solution: The Henderson-Hasselbalch Equation
The most common method for calculating the pH of a buffer solution is using the Henderson-Hasselbalch equation:
pH = pKa + log([A⁻]/[HA])
Where:
- pH: The pH of the buffer solution.
- pKa: The negative logarithm of the acid dissociation constant (Ka) of the weak acid. The pKa is a measure of the acid's strength; a lower pKa indicates a stronger acid.
- [A⁻]: The concentration of the conjugate base.
- [HA]: The concentration of the weak acid.
This equation highlights the relationship between the pH, the acid's strength (pKa), and the relative concentrations of the weak acid and its conjugate base. Let's break down how to use this equation effectively:
1. Identifying the Weak Acid and its Conjugate Base:
Accurately identifying the weak acid and its conjugate base is the first crucial step. For example, a buffer solution made from acetic acid (CH₃COOH) and sodium acetate (CH₃COONa) has acetic acid as the weak acid (HA) and acetate ion (CH₃COO⁻) as the conjugate base (A⁻).
2. Determining the pKa Value:
The pKa value can be found in various chemistry handbooks, databases, or online resources. It's a constant value for a given weak acid at a specific temperature. It's critical to use the pKa value corresponding to the temperature at which the buffer solution is prepared.
3. Measuring the Concentrations:
Precisely measuring the concentrations of the weak acid ([HA]) and its conjugate base ([A⁻]) is crucial. This typically involves using volumetric techniques such as preparing solutions with accurately measured volumes and concentrations. Units for concentration are typically molarity (moles per liter).
4. Applying the Henderson-Hasselbalch Equation:
Once you have the pKa and the concentrations, substitute these values into the Henderson-Hasselbalch equation to calculate the pH. A calculator capable of handling logarithms is essential for this calculation.
Example Calculation:
Let's consider a buffer solution made from 0.1 M acetic acid (pKa = 4.76) and 0.2 M sodium acetate.
pH = 4.76 + log(0.2 M / 0.1 M) pH = 4.76 + log(2) pH ≈ 4.76 + 0.30 pH ≈ 5.06
Therefore, the calculated pH of this buffer solution is approximately 5.06.
Measuring the pH of a Buffer Solution: Practical Techniques
While the Henderson-Hasselbalch equation provides a theoretical calculation, practical measurement offers a direct assessment of the buffer's pH. The most common method involves using a pH meter.
Using a pH Meter:
A pH meter is an electronic instrument used to measure the pH of a solution. The procedure involves:
-
Calibration: Before any measurement, the pH meter must be calibrated using standard buffer solutions of known pH (typically pH 4, 7, and 10). This ensures accurate and reliable readings.
-
Measurement: After calibration, carefully immerse the pH meter's electrode into the buffer solution, ensuring the electrode is completely submerged and free of air bubbles.
-
Reading: Allow the reading to stabilize before recording the pH value displayed on the meter. The pH meter should provide a stable reading within a few seconds to minutes, depending on the instrument and solution.
-
Cleaning: After each measurement, thoroughly clean the electrode to prevent contamination from the previous solution.
Other Methods:
While pH meters are the most accurate and commonly used method, other less precise methods exist, including pH indicator papers and solutions. These are suitable for approximate pH determinations but lack the precision of a pH meter.
Factors Affecting Buffer Capacity and pH
Several factors can influence the effectiveness of a buffer solution and its pH:
-
Temperature: The pKa of a weak acid is temperature-dependent, so changes in temperature can affect the pH of the buffer solution.
-
Ionic Strength: High ionic strength can influence the activity coefficients of the ions in the solution, leading to deviations from the ideal behavior predicted by the Henderson-Hasselbalch equation.
-
Concentration: The concentration of the weak acid and its conjugate base affects the buffer capacity. A higher concentration generally provides a greater buffer capacity, meaning the buffer can resist larger changes in pH.
Advanced Considerations: Buffer Capacity and Titration Curves
Buffer capacity refers to the amount of acid or base a buffer can absorb without a significant change in pH. A higher buffer capacity indicates a more robust buffer solution. This is directly related to the concentrations of the weak acid and its conjugate base. The closer the concentrations are to each other, the higher the buffer capacity will be, resulting in a flatter region around the pKa in the titration curve.
Understanding titration curves can further enhance comprehension of buffer behavior. A titration curve plots the pH of a solution against the volume of added titrant (acid or base). The buffer region is characterized by a relatively flat portion of the curve around the pKa of the weak acid. The slope of the curve in this region reflects the buffer capacity – a smaller slope indicates higher capacity.
Applications of pH Determination in Buffer Solutions
The ability to accurately determine the pH of buffer solutions is crucial across various scientific disciplines and industries:
-
Biochemistry and Molecular Biology: Enzyme assays and protein purification often rely on precise pH control, making pH measurement a critical step in these procedures.
-
Analytical Chemistry: Buffer solutions are fundamental in many analytical techniques, such as titrations and spectrophotometry, requiring accurate pH measurement for reliable results.
-
Environmental Science: Monitoring the pH of water bodies and soil samples is essential for assessing environmental health, often involving buffer solutions in sample preparation or analysis.
-
Food Science and Technology: Food preservation and processing utilize buffer solutions to maintain optimal pH ranges for product quality and stability.
Conclusion
Determining the pH of a buffer solution is essential in numerous scientific and industrial applications. While the Henderson-Hasselbalch equation provides a theoretical framework for calculating pH, using a pH meter is the most accurate and practical method for measuring the pH directly. Understanding the factors influencing buffer capacity and pH, alongside concepts like titration curves, ensures optimal use of buffer solutions and precise control over pH in various contexts. Careful attention to detail in both calculation and measurement ensures accurate results, contributing to successful outcomes in diverse fields.
Latest Posts
Latest Posts
-
Least Common Multiple Of 4 And 3
Mar 30, 2025
-
How Many Lines Of Symmetry Does A Regular Decagon Have
Mar 30, 2025
-
How Does An Igneous Rock Become A Sedimentary Rock
Mar 30, 2025
-
The Mass Of One Mole Of Carbon Dioxide Is
Mar 30, 2025
-
20 Is 85 Of What Number
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How To Find Ph Of Buffer Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
