What Subatomic Particles Make Up An Atom
listenit
Mar 27, 2025 · 6 min read
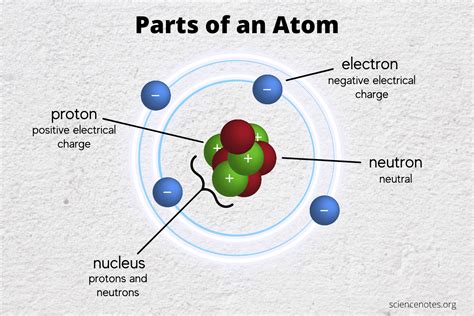
Table of Contents
What Subatomic Particles Make Up an Atom? A Deep Dive into the Building Blocks of Matter
Understanding the fundamental building blocks of matter is a journey into the fascinating world of subatomic particles. While the concept of an atom as the smallest unit of matter once held sway, we now know that atoms are complex structures composed of even smaller particles. This article will delve into the intricacies of these subatomic particles, exploring their properties, interactions, and the role they play in shaping the universe as we know it.
The Core Components: Protons, Neutrons, and Electrons
The atom's structure can be visualized as a miniature solar system. At the center lies the nucleus, a dense region containing two types of particles: protons and neutrons. These particles are collectively known as nucleons. Surrounding the nucleus is a cloud of electrons, which occupy specific energy levels or orbitals.
Protons: The Positively Charged Heart
Protons are positively charged particles, carrying a charge of +1 elementary charge. Their mass is approximately 1,836 times greater than that of an electron. The number of protons in an atom's nucleus defines its atomic number, a fundamental property that determines the element to which the atom belongs. For example, hydrogen has one proton (atomic number 1), helium has two (atomic number 2), and so on. The number of protons dictates the atom's chemical properties and its place on the periodic table.
Neutrons: The Neutral Stabilizers
Neutrons, as their name suggests, carry no net electric charge. Their mass is slightly larger than that of a proton. While not directly involved in chemical reactions, neutrons play a crucial role in nuclear stability. The number of neutrons in an atom's nucleus is known as the neutron number. For most elements, the number of neutrons is roughly equal to the number of protons, ensuring a stable nucleus. However, isotopes exist where the neutron number varies, leading to different properties and stability. Too few or too many neutrons can result in an unstable nucleus prone to radioactive decay.
Electrons: The Negatively Charged Orbiters
Electrons are negatively charged particles, carrying a charge of -1 elementary charge. Their mass is significantly smaller than that of protons or neutrons. Electrons orbit the nucleus in specific energy levels, or shells, described by quantum mechanics. These shells dictate the atom's chemical behavior. The outermost shell, also known as the valence shell, contains electrons that participate in chemical bonding, forming molecules and compounds. The number of electrons in an atom is typically equal to the number of protons, resulting in a neutral atom. However, atoms can gain or lose electrons, forming ions with a net positive (cation) or negative (anion) charge.
Delving Deeper: Quarks and Leptons – The Fundamental Particles
While protons, neutrons, and electrons were once considered fundamental particles, further research revealed a more complex reality. These particles are actually composed of even smaller, fundamental particles governed by the Standard Model of particle physics.
Quarks: The Constituents of Protons and Neutrons
Protons and neutrons are not elementary but are made up of quarks. Quarks are fundamental particles that come in six flavors: up, down, charm, strange, top, and bottom. Each quark has a fractional electric charge (+2/3 or -1/3 elementary charge).
- Protons: Composed of two up quarks and one down quark (uud). The combined charge is +2/3 + 2/3 - 1/3 = +1.
- Neutrons: Composed of one up quark and two down quarks (udd). The combined charge is +2/3 - 1/3 - 1/3 = 0.
Quarks are held together by the strong force, mediated by gluons. The strong force is incredibly powerful at short distances, binding quarks together within protons and neutrons.
Leptons: A Separate Family of Fundamental Particles
Electrons belong to a family of particles called leptons. Leptons are fundamental particles that do not experience the strong force. Besides electrons, other leptons include muons, tau particles, and their associated neutrinos. Neutrinos are incredibly elusive particles with very little mass and weak interaction with other matter.
The Forces at Play: Understanding Interactions
The behavior of subatomic particles is governed by four fundamental forces:
- Strong Force: The strongest of the four forces, responsible for binding quarks together within protons and neutrons and holding the nucleus together.
- Electromagnetic Force: Responsible for interactions between charged particles, such as the attraction between electrons and protons. This force governs chemical bonding and many everyday phenomena.
- Weak Force: Responsible for radioactive decay, allowing for the transformation of one type of quark into another.
- Gravitational Force: The weakest of the four forces, but significant on a macroscopic scale. It governs the attraction between objects with mass.
Isotopes and Their Significance
As mentioned earlier, isotopes are atoms of the same element with the same number of protons but differing numbers of neutrons. This variation in neutron number affects the atom's stability and mass. Some isotopes are stable, while others are radioactive, undergoing decay to achieve a more stable configuration. Radioactive isotopes have various applications in medicine, industry, and research. For example, Carbon-14 dating utilizes the radioactive decay of Carbon-14 to determine the age of organic materials.
Antimatter: The Mirror Image
For every particle, there exists a corresponding antiparticle with the same mass but opposite charge and other quantum numbers. When a particle and its antiparticle collide, they annihilate each other, releasing a burst of energy. The existence of antimatter adds another layer of complexity to our understanding of subatomic particles and the universe's origins.
Beyond the Standard Model: Unanswered Questions
While the Standard Model of particle physics successfully describes the interactions of most known subatomic particles, some unanswered questions remain. For example, the model doesn't fully explain dark matter and dark energy, which constitute a significant portion of the universe's mass-energy content. Ongoing research continues to explore these mysteries and further refine our understanding of the fundamental building blocks of matter.
Conclusion: A Journey into the Infinitesimally Small
The exploration of subatomic particles is a continuous journey of discovery, revealing the incredible complexity hidden within the seemingly simple atom. From the positively charged protons and neutral neutrons in the nucleus to the negatively charged electrons orbiting it, and further down to the quarks and leptons that compose them, understanding these particles unveils the fundamental forces shaping our universe. While much has been learned, mysteries remain, driving ongoing research and pushing the boundaries of our knowledge about the universe’s most basic components. The quest to understand the subatomic realm is a testament to human curiosity and our relentless pursuit of knowledge about the very fabric of reality.
Latest Posts
Related Post
Thank you for visiting our website which covers about What Subatomic Particles Make Up An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
