What Are Two Functional Groups Found In Amino Acids
listenit
Mar 27, 2025 · 7 min read
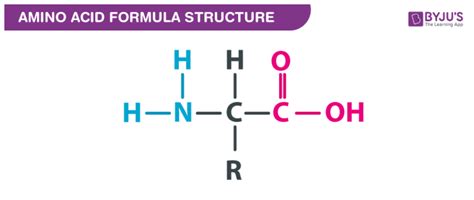
Table of Contents
What are the Two Functional Groups Found in Amino Acids?
Amino acids are the fundamental building blocks of proteins, the workhorses of life. Their remarkable versatility stems from their unique chemical structure, which invariably includes two crucial functional groups: the carboxyl group (-COOH) and the amino group (-NH2). Understanding the properties and roles of these groups is crucial to grasping the diverse functions of amino acids and proteins. This comprehensive article will delve deep into the characteristics of these functional groups, exploring their individual contributions and their synergistic interplay within the amino acid molecule.
The Carboxyl Group (-COOH): The Acidic Backbone
The carboxyl group, also known as a carboxylic acid group, is a defining feature of amino acids. It consists of a carbon atom double-bonded to an oxygen atom and single-bonded to a hydroxyl group (-OH). This arrangement gives the carboxyl group several key properties:
1. Acidity: The Proton Donor
The defining characteristic of the carboxyl group is its acidity. The oxygen atoms are highly electronegative, drawing electron density away from the hydroxyl group's hydrogen atom. This weakens the bond between the hydrogen and the oxygen, making the hydrogen relatively easy to lose as a proton (H+). This proton donation is what gives the carboxyl group its acidic nature. The deprotonated form of the carboxyl group is a carboxylate ion (-COO-), carrying a negative charge.
2. Hydrogen Bonding Capabilities: A Crucial Interaction
The carboxyl group's ability to form hydrogen bonds is paramount to its function. The oxygen atoms, with their partial negative charges, can act as hydrogen bond acceptors, attracting hydrogen atoms from other molecules containing electronegative atoms like oxygen or nitrogen. Similarly, the hydroxyl group's hydrogen atom can act as a hydrogen bond donor. This hydrogen bonding contributes significantly to the secondary, tertiary, and quaternary structures of proteins, influencing their overall shape and function.
3. Reaction with Other Molecules: Versatility in Synthesis
The carboxyl group's reactivity allows it to participate in various chemical reactions crucial for biological processes. It can react with amines to form amide bonds (peptide bonds), a key reaction in protein synthesis. The carboxyl group can also participate in esterification reactions, forming esters with alcohols. These reactions are vital for diverse biological functions, including the synthesis of lipids and other biomolecules.
4. pH Dependence: Influence of the Environment
The protonation state of the carboxyl group is highly pH-dependent. In acidic environments (low pH), the carboxyl group remains largely protonated (-COOH). As the pH increases (becomes more basic), the carboxyl group progressively loses its proton, becoming deprotonated (-COO-). This pH-dependent behavior is crucial for regulating the activity of proteins and enzymes.
The Amino Group (-NH2): The Basic Building Block
The amino group, an essential component of amino acids, comprises a nitrogen atom bonded to two hydrogen atoms. Its characteristics complement those of the carboxyl group, leading to the unique amphoteric nature of amino acids.
1. Basicity: The Proton Acceptor
The amino group is basic, meaning it readily accepts protons (H+). The nitrogen atom has a lone pair of electrons that can attract and bond with a proton, forming an ammonium ion (-NH3+). This protonation increases the positive charge on the nitrogen atom.
2. Hydrogen Bonding: Structure and Function
Similar to the carboxyl group, the amino group participates extensively in hydrogen bonding. The nitrogen atom, with its lone pair of electrons, acts as a hydrogen bond acceptor, while the hydrogen atoms can serve as hydrogen bond donors. These interactions contribute to the protein's three-dimensional structure and stability. It's worth noting that the positively charged ammonium ion (-NH3+) forms even stronger hydrogen bonds.
3. Reaction with Other Molecules: Peptide Bond Formation
The amino group's reactivity is crucial for protein synthesis. It reacts with the carboxyl group of another amino acid to form a peptide bond, creating the backbone of the polypeptide chain. This condensation reaction releases a water molecule. The peptide bond is a type of amide bond and is critical for protein structure and function.
4. pH Dependence: Balancing the Charge
Like the carboxyl group, the amino group's protonation state is strongly pH-dependent. In acidic conditions, the amino group remains predominantly protonated (-NH3+), while in basic conditions, it tends to lose a proton and become a neutral amino group (-NH2). This pH sensitivity allows amino acids to act as buffers, resisting changes in pH.
The Zwitterion Form: The Unique Amphoteric Nature
The simultaneous presence of both the acidic carboxyl group and the basic amino group gives amino acids their unique amphoteric character. At a specific pH value, known as the isoelectric point (pI), amino acids exist as zwitterions. A zwitterion is a molecule with both a positive and a negative charge, resulting from the carboxyl group losing a proton and the amino group accepting a proton. This neutral overall charge significantly affects the amino acid's behavior in solution and its interactions with other molecules. The pI value varies depending on the specific amino acid's side chain.
The Side Chain (R-Group): Adding Diversity
While the carboxyl and amino groups are common to all amino acids, the side chain (R-group) distinguishes the 20 standard amino acids from each other. This R-group can be nonpolar, polar uncharged, polar charged (acidic or basic), or even contain special chemical groups like aromatic rings, hydroxyl groups, sulfhydryl groups, or imidazole rings. The properties of the R-group significantly influence the overall characteristics of the amino acid and the resulting protein's structure and function. The variations in R-group properties account for the immense diversity and functionality of proteins.
The Interplay of Functional Groups: Building Protein Structure
The precise interplay between the carboxyl and amino groups is fundamental to the construction of protein structure. The formation of peptide bonds between the carboxyl group of one amino acid and the amino group of another is the cornerstone of protein synthesis. This process creates a linear chain of amino acids, the primary structure of a protein.
The secondary structure, involving alpha-helices and beta-sheets, arises from hydrogen bonding between the carbonyl oxygen of one amino acid and the amide hydrogen of another. These hydrogen bonds, which are significantly impacted by the carboxyl and amino groups, stabilize these secondary structures.
The tertiary structure, the overall three-dimensional folding of the polypeptide chain, is determined by numerous interactions, including hydrogen bonds, hydrophobic interactions, disulfide bridges, ionic bonds, and van der Waals forces. The carboxyl and amino groups play a vital role in hydrogen bonding and the formation of ionic bonds, shaping the tertiary structure. Further stabilizing interactions among side chains contribute to the complex three-dimensional architecture.
The quaternary structure, present in multi-subunit proteins, is the arrangement of multiple polypeptide chains. Interactions between the carboxyl and amino groups of different subunits, alongside side-chain interactions, contribute to the stabilization of this higher-order structure.
The Importance of Amino Acid Structure and Function in Biological Processes
The diverse properties of amino acids arising from their carboxyl and amino groups are essential for a wide range of biological functions:
-
Enzyme Catalysis: Many enzymes rely on the specific arrangement of amino acid side chains, including those with carboxyl and amino groups, to create active sites that catalyze biochemical reactions. The precise placement of these functional groups is crucial for substrate binding and catalysis.
-
Signal Transduction: Amino acids act as signaling molecules, participating in various cellular processes. The charged nature of the carboxyl and amino groups plays a critical role in their interactions with cell receptors.
-
Structural Support: Proteins such as collagen and keratin, which provide structural support in tissues, depend heavily on interactions between the carboxyl and amino groups, maintaining their stability and strength.
-
Transport and Storage: Hemoglobin, responsible for oxygen transport, and ferritin, responsible for iron storage, utilize the specific properties of amino acids to facilitate their roles. These proteins rely on intricate interactions involving the carboxyl and amino groups.
Conclusion: The Foundation of Life
The carboxyl and amino groups are the cornerstones of amino acid structure and functionality. Their complementary properties, including acidity, basicity, and hydrogen bonding capacity, enable them to participate in a wide variety of biological processes. The unique amphoteric nature of amino acids, due to these two functional groups, influences their behavior in solution and their interactions with other molecules. Understanding the interplay of these functional groups is essential to comprehending the complexities of protein structure, function, and their vital roles in life's processes. Their combined effect generates the remarkable diversity and functionality of proteins, highlighting their fundamental importance in all living organisms. From simple structures to complex enzymatic machinery, the interplay between these two seemingly simple groups underpins the incredible complexity of life itself.
Latest Posts
Latest Posts
-
What Percent Of 186 Is 93
Mar 31, 2025
-
How To Find The Volume Of A Hexagonal Prism
Mar 31, 2025
-
How Much Is 64 Fl Oz
Mar 31, 2025
-
What Is The Electron Configuration Of Lithium
Mar 31, 2025
-
How To Integrate 1 X 2 2
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Are Two Functional Groups Found In Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
