What Is The Electron Configuration Of Lithium
listenit
Mar 31, 2025 · 8 min read
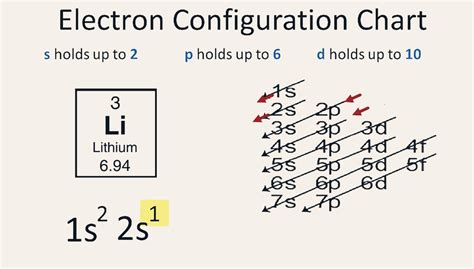
Table of Contents
What is the Electron Configuration of Lithium? A Deep Dive into Atomic Structure
Lithium, the lightest of the alkali metals, holds a fascinating place in the periodic table. Understanding its electron configuration is key to unlocking its unique chemical properties and behavior. This comprehensive guide will delve into the intricacies of lithium's electron configuration, exploring its significance and implications. We'll cover the basics, explain the underlying principles, and even explore some advanced concepts related to its electronic structure.
Understanding Electron Configuration
Before we dive into lithium's specific configuration, let's establish a foundational understanding of what electron configuration means. An electron configuration is a description of how electrons are distributed in the various energy levels and sublevels within an atom. It's a shorthand notation that reveals the arrangement of electrons, dictated by the principles of quantum mechanics. This arrangement dictates an atom's chemical properties and its ability to form bonds with other atoms.
The configuration is typically written as a series of numbers and letters, reflecting the principal quantum number (n), the azimuthal quantum number (l), and the number of electrons in each sublevel.
-
Principal Quantum Number (n): Represents the energy level or shell. n can be any positive integer (1, 2, 3, ...). Higher n values indicate higher energy levels and greater distance from the nucleus.
-
Azimuthal Quantum Number (l): Represents the sublevel or subshell within a principal energy level. l can range from 0 to n-1. Each value of l corresponds to a specific subshell:
- l = 0: s subshell (spherical shape, holds a maximum of 2 electrons)
- l = 1: p subshell (dumbbell shape, holds a maximum of 6 electrons)
- l = 2: d subshell (more complex shape, holds a maximum of 10 electrons)
- l = 3: f subshell (even more complex shape, holds a maximum of 14 electrons)
-
Electron Count: The superscript following each subshell designation indicates the number of electrons occupying that specific subshell.
Determining Lithium's Electron Configuration
Lithium (Li) has an atomic number of 3, meaning it possesses 3 protons in its nucleus and, in a neutral atom, 3 electrons surrounding the nucleus. To determine its electron configuration, we follow the Aufbau principle, which states that electrons fill the lowest energy levels first. We also consider the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms), and Hund's rule, which states that electrons will individually occupy each orbital within a subshell before doubling up.
Following these rules, we fill the energy levels systematically:
-
The first energy level (n=1) contains only the 1s subshell. This subshell can hold a maximum of two electrons. Therefore, lithium's two lowest-energy electrons will fill the 1s subshell.
-
The second energy level (n=2) contains the 2s and 2p subshells. After filling the 1s subshell, the remaining electron will occupy the lowest energy subshell in the second energy level, which is the 2s subshell.
Therefore, the electron configuration of lithium is 1s²2s¹. This means that there are two electrons in the 1s subshell and one electron in the 2s subshell.
Visualizing Lithium's Electron Configuration
It's helpful to visualize the electron configuration. We can represent this using orbital diagrams, which show the individual orbitals within each subshell and how the electrons fill them.
For lithium, the orbital diagram would look like this:
1s: ↑↓ 2s: ↑
Each arrow represents an electron, and the up and down arrows indicate opposite spins (according to the Pauli exclusion principle). The 1s subshell is full, while the 2s subshell contains only one electron.
Significance of Lithium's Electron Configuration
Lithium's electron configuration is crucial in understanding its chemical behavior. The single electron in the 2s orbital is relatively loosely held and easily lost. This readily available electron makes lithium highly reactive, readily participating in chemical reactions to achieve a stable, filled outer shell (like the noble gases). This explains why lithium readily forms +1 ions (Li⁺), losing its single valence electron to achieve a stable electron configuration similar to helium (1s²).
Lithium's Reactivity and Chemical Bonding
The ease with which lithium loses its valence electron directly impacts its reactivity and the types of chemical bonds it forms. Lithium readily forms ionic bonds with nonmetals, such as chlorine, where it donates its valence electron to the chlorine atom. This electron transfer results in the formation of lithium chloride (LiCl), an ionic compound with a strong electrostatic attraction between the positively charged lithium ion and the negatively charged chloride ion.
Lithium can also participate in covalent bonding, although it is less common than ionic bonding. In covalent bonds, lithium shares its valence electron with another atom. However, due to its low electronegativity (the ability of an atom to attract electrons), the covalent bonds lithium forms are usually polar, with the shared electron pair being closer to the more electronegative atom.
Lithium's Applications: A Consequence of its Electronic Structure
The unique electronic structure of lithium, specifically its readily available valence electron, leads to a wide range of applications. Lithium's reactivity makes it useful in various industrial processes, including:
-
Lithium-ion batteries: The ability of lithium to easily lose and gain electrons makes it ideal for use in rechargeable batteries. Lithium-ion batteries are used in a vast array of devices, from portable electronics to electric vehicles, due to their high energy density and relatively long lifespan.
-
Lubricants: Lithium-based greases are used as high-temperature lubricants due to their excellent thermal stability and resistance to oxidation.
-
Alloys: Lithium alloys are used to improve the strength and other mechanical properties of certain metals.
-
Glass and ceramics: Lithium compounds are added to glass and ceramic materials to improve their thermal and chemical resistance.
-
Medical applications: Lithium salts have been used in the treatment of bipolar disorder. The exact mechanism of action is not fully understood, but it's believed to involve interactions with neurotransmitters in the brain.
Beyond the Basics: Excited States and Spectroscopy
While the 1s²2s¹ configuration represents lithium in its ground state (lowest energy level), it can also exist in excited states. When lithium atoms absorb energy (e.g., heat or light), the electron in the 2s orbital can be promoted to a higher energy level, such as the 2p or even higher orbitals. These excited states are less stable and will eventually return to the ground state, emitting energy in the process.
This energy emission forms the basis of atomic spectroscopy, a technique used to identify elements based on their characteristic emission spectra. The specific wavelengths of light emitted by lithium atoms when they return from excited states to the ground state provide a unique fingerprint that allows for its identification. Analyzing these spectral lines offers crucial insights into the electronic transitions within lithium atoms and the energy differences between its various energy levels.
Advanced Concepts: Effective Nuclear Charge and Shielding
The electron configuration doesn't fully capture the complexities of atomic structure. The interaction between electrons and the nucleus is not a simple Coulombic interaction. Concepts like effective nuclear charge and shielding effect are crucial for a more complete understanding.
Effective nuclear charge (Z<sub>eff</sub>) refers to the net positive charge experienced by an electron in a multi-electron atom. It's less than the actual nuclear charge (Z) because of the shielding effect of other electrons. Inner electrons shield outer electrons from the full positive charge of the nucleus, reducing the attractive force experienced by the outer electrons. In lithium, the 1s electrons effectively shield the 2s electron from the full positive charge of the three protons in the nucleus. The 2s electron therefore experiences a reduced effective nuclear charge.
Shielding effect is the reduction in the effective nuclear charge on the outer electrons due to the presence of inner electrons. The inner electrons repel the outer electrons, reducing the attraction between the outer electrons and the nucleus. The greater the number of inner electrons, the greater the shielding effect.
These concepts are crucial for understanding trends in ionization energy, atomic radius, and other periodic properties. The relatively low effective nuclear charge experienced by lithium's 2s electron explains its low ionization energy and its high reactivity.
Conclusion: Lithium's Simple Yet Significant Configuration
While seemingly simple, the electron configuration of lithium (1s²2s¹) holds the key to understanding its chemical behavior, reactivity, and its myriad applications. Its single valence electron, shielded by the inner shell electrons, accounts for its readiness to participate in chemical reactions and the formation of ionic compounds. This understanding, coupled with more advanced concepts like effective nuclear charge and shielding, provides a comprehensive picture of this fascinating element's atomic structure and its place in the world around us. From its role in high-tech batteries to its therapeutic uses, the significance of lithium’s electron configuration extends far beyond the confines of the chemistry lab.
Latest Posts
Latest Posts
-
Lewis Acid Vs Bronsted Lowry Acid
Apr 01, 2025
-
What Is Square Root Of 63
Apr 01, 2025
-
Least Common Multiple 2 And 4
Apr 01, 2025
-
How Does Igneous Rock Become Metamorphic
Apr 01, 2025
-
How Does Friction Affect The Motion Of Objects
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Lithium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
