Lewis Acid Vs Bronsted Lowry Acid
listenit
Apr 01, 2025 · 6 min read
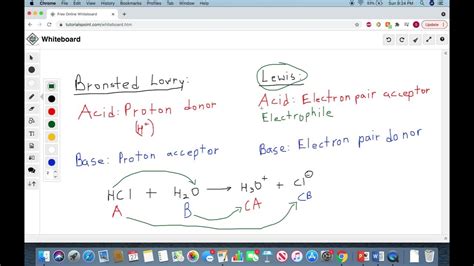
Table of Contents
Lewis Acid vs. Brønsted-Lowry Acid: A Comprehensive Comparison
Understanding the differences and similarities between Lewis acids and Brønsted-Lowry acids is crucial for a firm grasp of acid-base chemistry. While both definitions describe substances that can act as acids, they approach the concept from different perspectives, leading to a broader understanding of acid-base reactions. This article delves deep into the characteristics, examples, and applications of both types of acids, providing a comprehensive comparison that clarifies their distinctions and overlaps.
Defining Acids: Two Perspectives
The concept of "acid" has evolved throughout the history of chemistry. Initially, acids were defined based on observable properties like sour taste and the ability to react with certain metals. However, these observations are insufficient to encompass the full scope of acidic behavior. Two major theoretical frameworks provide a more comprehensive understanding:
Brønsted-Lowry Acids: Proton Donors
The Brønsted-Lowry theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, defines an acid as a substance that donates a proton (H⁺). This definition centers on the transfer of a hydrogen ion, highlighting the crucial role of protons in acid-base reactions. A Brønsted-Lowry acid must possess at least one hydrogen atom that can be released as a proton.
Key characteristics of Brønsted-Lowry acids:
- Proton donation: This is the defining feature. The acid donates a proton to a base, which accepts it.
- Conjugate acid-base pairs: When a Brønsted-Lowry acid donates a proton, it forms its conjugate base. The conjugate base is the species remaining after the proton is released. The acid and its conjugate base differ by only a proton.
- Water as a solvent: Many Brønsted-Lowry acid-base reactions occur in aqueous solutions, with water often acting as either an acid or a base.
Examples of Brønsted-Lowry acids:
- Hydrochloric acid (HCl): HCl → H⁺ + Cl⁻
- Sulfuric acid (H₂SO₄): H₂SO₄ → H⁺ + HSO₄⁻ (first proton donation)
- Acetic acid (CH₃COOH): CH₃COOH → H⁺ + CH₃COO⁻
- Ammonium ion (NH₄⁺): NH₄⁺ → H⁺ + NH₃
Lewis Acids: Electron Pair Acceptors
Gilbert N. Lewis broadened the definition of acids and bases in 1923, introducing a more encompassing concept. A Lewis acid is defined as a substance that can accept a pair of electrons. This definition doesn't restrict acids to proton donors; instead, it focuses on the ability to form a coordinate covalent bond by accepting electron density. This broader perspective incorporates many substances that wouldn't be considered acids under the Brønsted-Lowry definition.
Key characteristics of Lewis acids:
- Electron pair acceptance: Lewis acids possess an empty orbital capable of accepting a pair of electrons from a Lewis base.
- Coordinate covalent bond formation: The interaction between a Lewis acid and a Lewis base involves the formation of a coordinate covalent bond, where both electrons in the bond are donated by the base.
- Variety of chemical species: Lewis acids encompass a wide range of compounds, including metal cations, molecules with incomplete octets, and molecules with polar bonds.
Examples of Lewis acids:
- Boron trifluoride (BF₃): BF₃ has an incomplete octet and readily accepts an electron pair.
- Aluminum chloride (AlCl₃): Similar to BF₃, AlCl₃ can accept an electron pair to complete its octet.
- Iron(III) ion (Fe³⁺): Metal cations with high charge density act as Lewis acids due to their ability to attract electron pairs.
- Carbon dioxide (CO₂): The carbon atom in CO₂ has a partial positive charge and can accept electron pairs.
The Overlap and Distinction: Brønsted-Lowry vs. Lewis Acids
All Brønsted-Lowry acids are also Lewis acids, but not all Lewis acids are Brønsted-Lowry acids. This is a crucial distinction.
Why are all Brønsted-Lowry acids also Lewis acids?
When a Brønsted-Lowry acid donates a proton (H⁺), it's essentially accepting a pair of electrons from the base. The proton, lacking electrons, accepts the electron pair from the base to form a bond. This electron pair acceptance aligns precisely with the Lewis acid definition. Therefore, the Brønsted-Lowry definition is a subset of the Lewis definition.
Why aren't all Lewis acids Brønsted-Lowry acids?
The Lewis definition is broader because it doesn't require the presence of a proton. Many Lewis acids, like BF₃ or AlCl₃, cannot donate protons, yet they readily accept electron pairs from Lewis bases. This illustrates the expanded scope of the Lewis acid-base theory.
Applications and Significance
The understanding of both Brønsted-Lowry and Lewis acids is essential across diverse chemical applications:
Brønsted-Lowry Acids in Everyday Life and Industry:
- Acid-base titrations: These are crucial analytical techniques used to determine the concentration of unknown solutions.
- pH regulation: Brønsted-Lowry acids are essential in maintaining the desired pH in various industrial processes and biological systems.
- Food and beverage industry: Acids like citric acid and acetic acid are commonly used as preservatives and flavor enhancers.
- Pharmaceuticals: Many drugs and medications involve Brønsted-Lowry acids or bases.
Lewis Acids in Catalysis and Synthesis:
- Organic synthesis: Lewis acids are widely employed as catalysts in a vast range of organic reactions, facilitating bond formation and rearrangements. For example, Friedel-Crafts alkylation and acylation reactions rely heavily on Lewis acids like AlCl₃.
- Polymer chemistry: Lewis acids play a role in polymerization reactions, influencing the structure and properties of the resulting polymers.
- Material science: The synthesis and modification of numerous materials benefit from the use of Lewis acids, allowing for precise control over the structure and properties of the final product.
Advanced Concepts and Extensions
The Lewis and Brønsted-Lowry theories, while providing powerful frameworks, are not without their limitations. Research continues to expand our understanding of acid-base chemistry, encompassing more complex situations:
- Hard and soft acids and bases (HSAB theory): This theory categorizes Lewis acids and bases based on their hardness and softness, influencing their reactivity and selectivity. Hard acids prefer to react with hard bases, and soft acids prefer soft bases.
- Superacids: These are extremely strong acids that exceed the acidity of conventional strong acids like sulfuric acid. Many superacids are based on Lewis acid-Brønsted-Lowry acid combinations.
- Solvent effects: The solvent in which an acid-base reaction takes place significantly influences the reaction's equilibrium and rate. The solvent's ability to stabilize or destabilize ions affects the overall acidity or basicity.
Conclusion
The distinctions and overlaps between Lewis acids and Brønsted-Lowry acids highlight the evolution of our understanding of acid-base chemistry. While the Brønsted-Lowry definition focuses on proton transfer, the Lewis definition encompasses a broader range of electron-pair interactions. Both theories are crucial for comprehending a wide variety of chemical phenomena, with implications across various scientific disciplines and technological applications. Understanding both definitions allows for a more complete and nuanced appreciation of the dynamic world of acid-base reactions and their importance in chemistry and beyond. Furthermore, exploring advanced concepts such as HSAB theory and superacids deepens the understanding of these fundamental concepts, revealing the intricate and ever-evolving nature of acid-base chemistry.
Latest Posts
Latest Posts
-
Irrational Numbers Between 1 And 6
Apr 02, 2025
-
In A Hypertonic Solution A Cell Will
Apr 02, 2025
-
How Many Kilograms Are In 5000 Grams
Apr 02, 2025
-
Solve This Inequality 3b 7 32
Apr 02, 2025
-
Number Of Valence Electrons In Ar
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Lewis Acid Vs Bronsted Lowry Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
