How To Find Moles Of Naoh
listenit
Apr 01, 2025 · 5 min read
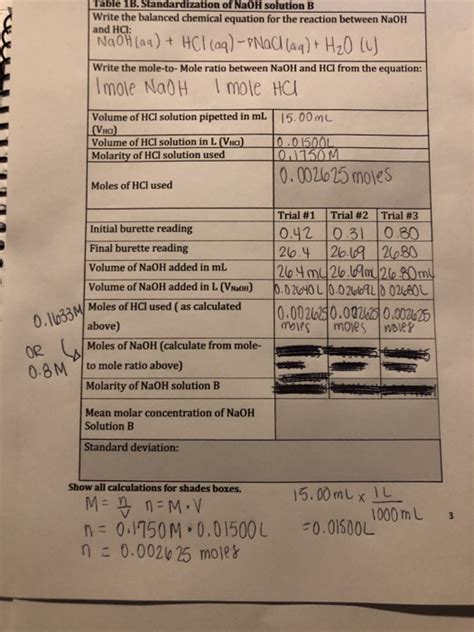
Table of Contents
How to Find Moles of NaOH: A Comprehensive Guide
Determining the number of moles of sodium hydroxide (NaOH), a common strong base used in various chemical applications, is a crucial skill in chemistry. This comprehensive guide will explore different methods for calculating moles of NaOH, covering various scenarios and considerations. Understanding these methods is essential for accurate stoichiometric calculations and successful experimental work.
Understanding Moles and Molar Mass
Before diving into the methods, let's solidify our understanding of fundamental concepts.
What is a Mole?
A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of particles (atoms, molecules, ions, etc.). It's a convenient way to express the amount of a substance.
What is Molar Mass?
Molar mass is the mass of one mole of a substance. It's expressed in grams per mole (g/mol). For NaOH, the molar mass is calculated by adding the atomic masses of its constituent elements:
- Na (Sodium): 22.99 g/mol
- O (Oxygen): 16.00 g/mol
- H (Hydrogen): 1.01 g/mol
Therefore, the molar mass of NaOH = 22.99 + 16.00 + 1.01 = 40.00 g/mol
Methods for Finding Moles of NaOH
Several methods exist for determining the number of moles of NaOH, depending on the information available.
Method 1: Using Mass and Molar Mass
This is the most straightforward method when you know the mass of NaOH. The formula is:
Moles (mol) = Mass (g) / Molar Mass (g/mol)
Example: You have 5 grams of NaOH. To find the number of moles:
Moles = 5 g / 40.00 g/mol = 0.125 mol
Therefore, 5 grams of NaOH contains 0.125 moles.
Method 2: Using Molarity and Volume
This method is applicable when you know the concentration (molarity) and volume of an NaOH solution. Molarity is expressed as moles per liter (mol/L). The formula is:
Moles (mol) = Molarity (mol/L) x Volume (L)
Example: You have 250 mL of a 0.5 M NaOH solution. First, convert the volume to liters: 250 mL = 0.25 L. Then calculate the number of moles:
Moles = 0.5 mol/L x 0.25 L = 0.125 mol
Thus, 250 mL of a 0.5 M NaOH solution contains 0.125 moles.
Method 3: Using Titration Data
Titration is a common laboratory technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration (the titrant). If you titrate NaOH with a standard solution (e.g., a standardized HCl solution), you can calculate the moles of NaOH using the stoichiometry of the reaction and the titration data.
Example: Let's say you titrated 25 mL of an unknown NaOH solution with 0.1 M HCl. The endpoint was reached after adding 20 mL of HCl. The balanced chemical equation for the reaction is:
NaOH + HCl → NaCl + H₂O
From the stoichiometry, the mole ratio of NaOH to HCl is 1:1. First, calculate the moles of HCl used:
Moles of HCl = 0.1 mol/L x 0.020 L = 0.002 mol
Since the mole ratio is 1:1, the moles of NaOH are equal to the moles of HCl:
Moles of NaOH = 0.002 mol
Therefore, the 25 mL sample of NaOH solution contained 0.002 moles of NaOH.
Method 4: Using Percent Composition and Mass
If you know the percent composition of NaOH in a sample and the total mass of the sample, you can calculate the moles of NaOH.
Example: Assume you have a 100g sample that is 80% NaOH. First, calculate the mass of NaOH in the sample:
Mass of NaOH = 100g x 0.80 = 80g
Then, use the mass and molar mass to calculate moles:
Moles of NaOH = 80g / 40.00 g/mol = 2 mol
Practical Considerations and Sources of Error
Several factors can influence the accuracy of your NaOH mole calculations.
-
Purity of NaOH: Commercial NaOH often contains impurities. If using a non-analytical grade NaOH, consider the purity percentage while calculating the actual moles of NaOH.
-
Accuracy of measurements: Inaccurate measurements of mass or volume will lead to errors in the calculated number of moles. Ensure that you use calibrated equipment and proper measurement techniques.
-
Temperature effects: The molarity of solutions can change with temperature. If performing calculations based on molarity, consider the temperature of the solution.
-
Reaction completion: In titrations, ensure that the reaction is complete before determining the endpoint. Incomplete reactions lead to inaccurate results.
-
Carbon dioxide absorption: NaOH is hygroscopic (absorbs moisture from the air) and reacts with atmospheric CO2. This can affect the accuracy of mass measurements and molarity calculations. Store NaOH properly to minimize these effects.
Advanced Applications and Related Concepts
The concepts described above are fundamental to various chemical calculations and applications.
-
Stoichiometry: Knowing the moles of NaOH is crucial for performing stoichiometric calculations, determining the amount of reactants needed or products formed in chemical reactions involving NaOH.
-
Solution preparation: Accurately calculating moles is essential when preparing solutions of a specific molarity.
-
Acid-base reactions: Understanding the moles of NaOH is vital for working with acid-base reactions, particularly in titrations and pH calculations.
-
Chemical kinetics: In reaction rate studies, knowing the concentration (and thus moles) of reactants, including NaOH, is essential for analyzing reaction rates and determining reaction orders.
Conclusion
Calculating the number of moles of NaOH is a fundamental skill in chemistry with wide-ranging applications. This guide has outlined various methods for calculating moles, discussed essential concepts, and addressed practical considerations and sources of error. By mastering these methods and understanding the associated factors, you can confidently perform chemical calculations involving NaOH and other substances, leading to accurate experimental results and a deeper understanding of chemical principles. Remember to always prioritize accurate measurements and proper experimental techniques for reliable results.
Latest Posts
Latest Posts
-
Instantaneous Rate Of Change Vs Average Rate Of Change
Apr 02, 2025
-
How Many D Orbitals Can Be In An Energy Level
Apr 02, 2025
-
Log Base 2 X 2 Graph
Apr 02, 2025
-
X 3 2x 2 5x 6
Apr 02, 2025
-
Is Boiling Water A Chemical Reaction
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How To Find Moles Of Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
