How To Find Concentration In Titration
listenit
Mar 23, 2025 · 5 min read
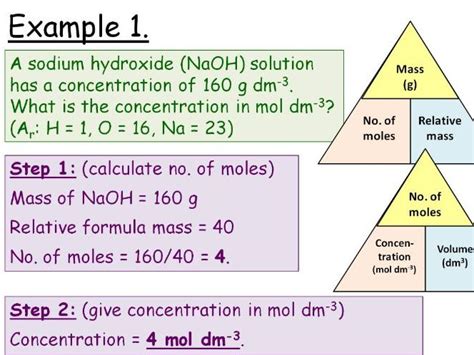
Table of Contents
How to Find Concentration in Titration: A Comprehensive Guide
Titration is a crucial analytical technique used to determine the unknown concentration of a solution, known as the analyte, by reacting it with a solution of known concentration, called the titrant. Mastering titration and accurately calculating the unknown concentration requires precision, attention to detail, and a solid understanding of the underlying chemistry. This comprehensive guide delves into the process of finding concentration in titration, covering everything from the fundamental principles to advanced troubleshooting techniques.
Understanding the Principles of Titration
Before diving into the calculations, let's establish a firm grasp of the core concepts:
What is Titration?
Titration involves the gradual addition of a titrant to an analyte until the reaction between them is complete. This "completion point," known as the equivalence point, is often indicated by a color change using an indicator. The volume of titrant used to reach the equivalence point is crucial for calculating the analyte's concentration.
Types of Titration:
Several types of titration exist, each suited for different analyte-titrant combinations:
-
Acid-Base Titration: This is the most common type, involving the reaction between an acid and a base. Strong acid-strong base titrations are straightforward, while weak acid-strong base or weak base-strong acid titrations require a more nuanced approach due to the influence of equilibrium constants.
-
Redox Titration: These titrations involve the transfer of electrons between the analyte and the titrant. Examples include permanganate titrations and iodometric titrations.
-
Complexometric Titration: These titrations involve the formation of a complex ion between the analyte and the titrant. EDTA titrations are a prime example, commonly used for determining the concentration of metal ions.
-
Precipitation Titration: In this type, the reaction between the analyte and the titrant leads to the formation of a precipitate. Silver nitrate titrations are a classic example used to determine halide ion concentrations.
Essential Equipment:
Accurate titration requires precise equipment:
- Burette: Used to deliver the titrant precisely.
- Pipette: Used to accurately measure the volume of the analyte.
- Conical Flask (Erlenmeyer Flask): To hold the analyte solution.
- Beaker: For preparing and mixing solutions.
- Indicator: To visually signal the equivalence point.
Calculating Concentration: A Step-by-Step Guide
The core of titration lies in the stoichiometric relationship between the analyte and the titrant. The calculation depends on the type of titration, but the general steps remain consistent:
1. Balanced Chemical Equation: Write a balanced chemical equation for the reaction between the analyte and the titrant. This equation is crucial for establishing the mole ratio between the two substances.
2. Moles of Titrant: Calculate the number of moles of titrant used. Use the following formula:
Moles of Titrant = Molarity of Titrant (mol/L) × Volume of Titrant (L)
Remember to convert the volume of titrant from milliliters (mL) to liters (L).
3. Mole Ratio: Determine the mole ratio between the analyte and the titrant from the balanced chemical equation. This ratio indicates the number of moles of analyte that react with one mole of titrant.
4. Moles of Analyte: Use the mole ratio to calculate the number of moles of analyte that reacted with the titrant.
Moles of Analyte = Moles of Titrant × (Mole Ratio of Analyte/Titrant)
5. Concentration of Analyte: Finally, calculate the concentration of the analyte using the following formula:
Molarity of Analyte (mol/L) = Moles of Analyte / Volume of Analyte (L)
Again, remember to convert the volume of analyte from mL to L.
Example: Acid-Base Titration
Let's illustrate this with an example of a strong acid-strong base titration:
Problem: 25.00 mL of an unknown concentration of HCl solution is titrated with 0.100 M NaOH solution. The equivalence point is reached after adding 20.00 mL of NaOH. Calculate the concentration of the HCl solution.
Solution:
-
Balanced Equation: HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
-
Moles of NaOH: Moles of NaOH = 0.100 mol/L × 0.0200 L = 0.00200 mol
-
Mole Ratio: From the balanced equation, the mole ratio of HCl to NaOH is 1:1.
-
Moles of HCl: Moles of HCl = 0.00200 mol (since the ratio is 1:1)
-
Concentration of HCl: Molarity of HCl = 0.00200 mol / 0.02500 L = 0.0800 M
Therefore, the concentration of the HCl solution is 0.0800 M.
Advanced Considerations and Troubleshooting
While the basic calculations are straightforward, several factors can influence the accuracy of titration results:
Indicator Choice:
The choice of indicator is critical. The indicator's pKa should be close to the pH at the equivalence point to ensure accurate determination. For strong acid-strong base titrations, phenolphthalein is commonly used. For weak acid-strong base titrations, an indicator with a different pKa range might be necessary.
Systematic Errors:
Several systematic errors can affect titration results:
- Parallax Error: Incorrect reading of the burette due to eye level not being aligned with the meniscus.
- Calibration Errors: Inaccurate calibration of the burette or pipette.
- Improper Mixing: Insufficient mixing of the analyte and titrant can lead to inaccurate readings.
Random Errors:
Random errors are inherent in any measurement and can be minimized by repeating the titration multiple times and calculating the average.
Dealing with Impurities:
If the analyte contains impurities, this will affect the calculated concentration. Techniques like standardization of the titrant or using a blank titration can help account for these impurities.
Enhancing Accuracy and Precision
Several strategies can help improve the accuracy and precision of titration:
- Multiple Trials: Performing multiple titrations and averaging the results reduces the impact of random errors.
- Proper Technique: Employing correct techniques in handling equipment and performing the titration is essential.
- Temperature Control: Maintaining a consistent temperature throughout the titration minimizes variations in reaction rates and equilibrium constants.
- Careful Observation: Paying close attention to the color change at the equivalence point improves accuracy.
Conclusion: Mastering the Art of Titration
Titration is a powerful analytical technique with widespread applications in various fields. By understanding the underlying principles, mastering the calculations, and employing proper techniques, you can accurately determine the unknown concentration of solutions. This guide provides a solid foundation for successfully performing titrations and interpreting the results. Remember that practice and attention to detail are key to achieving accurate and reliable results. Through meticulous execution and careful analysis, you can master the art of titration and unlock the secrets held within your solutions.
Latest Posts
Latest Posts
-
Is Color A Physical Or Chemical Change
Mar 25, 2025
-
Of The Following Which Atom Has The Largest Atomic Radius
Mar 25, 2025
-
How To Graph 3x Y 6
Mar 25, 2025
-
What Percent Is 21 Of 50
Mar 25, 2025
-
How Many Sigma Bonds Are Present In Acetylsalicylic Acid
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How To Find Concentration In Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
