Of The Following Which Atom Has The Largest Atomic Radius
listenit
Mar 25, 2025 · 5 min read
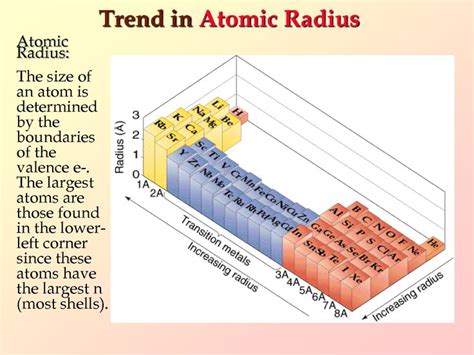
Table of Contents
Unveiling the Atomic Radius: A Deep Dive into Atomic Structure and Trends
Determining which atom possesses the largest atomic radius requires a nuanced understanding of atomic structure, periodic trends, and the forces governing electron behavior. While a simple answer might seem straightforward, a thorough exploration delves into the complexities of electron shielding, effective nuclear charge, and the quantum mechanical nature of the atom. This article will meticulously examine these factors, comparing various atoms and culminating in a definitive answer while providing a broader comprehension of atomic radii.
Understanding Atomic Radius
The atomic radius isn't a precisely defined, easily measurable quantity like the diameter of a marble. Instead, it represents the average distance between the nucleus and the outermost electron shell of an atom. It's crucial to understand that the electron cloud itself is probabilistic, meaning electrons don't orbit the nucleus in neat, predictable paths as depicted in simplistic models. Instead, their location is described by probability distributions, making the edge of the atom somewhat fuzzy.
Several methods exist for experimentally determining atomic radii, including X-ray crystallography and electron diffraction. These techniques provide estimates based on the distances between atoms in solid structures or the scattering patterns of electrons. While these methods offer valuable data, understanding the underlying principles that govern atomic size is paramount for predicting and comparing radii.
Factors Influencing Atomic Radius
Several key factors significantly influence an atom's radius:
-
Principal Quantum Number (n): This quantum number determines the energy level of an electron and, consequently, its average distance from the nucleus. As 'n' increases, the electron resides at a higher energy level, farther from the nucleus, resulting in a larger atomic radius. Atoms in higher periods (rows) of the periodic table generally have larger radii because their outermost electrons occupy higher energy levels.
-
Effective Nuclear Charge (Zeff): This is the net positive charge experienced by an electron after accounting for the shielding effect of other electrons. Electrons in inner shells shield outer electrons from the full positive charge of the nucleus. A higher Zeff pulls outer electrons closer to the nucleus, reducing the atomic radius. Conversely, a lower Zeff allows outer electrons to be further from the nucleus, increasing the atomic radius.
-
Shielding Effect: Inner electrons effectively shield outer electrons from the full attractive force of the nucleus. The greater the number of inner electrons, the more effective the shielding, and the weaker the attraction between the nucleus and the outermost electrons. This leads to a larger atomic radius.
-
Electron-Electron Repulsion: Electrons repel each other due to their like charges. In atoms with multiple electrons in the outermost shell, this repulsion can increase the atomic radius by pushing the electrons further apart.
Periodic Trends in Atomic Radius
Understanding periodic trends allows for a systematic comparison of atomic radii. These trends are crucial for predicting relative sizes without detailed calculations:
-
Across a Period (Left to Right): Atomic radius generally decreases as you move from left to right across a period. While the principal quantum number remains constant, the effective nuclear charge increases due to the addition of protons without a proportionate increase in shielding electrons. This stronger nuclear pull reduces the atomic radius.
-
Down a Group (Top to Bottom): Atomic radius generally increases as you move down a group. This is because each subsequent element adds another electron shell, increasing the principal quantum number (n) significantly. The increased distance of the outermost electrons from the nucleus outweighs the increase in Zeff, leading to a larger radius.
Comparing Atomic Radii: A Case Study
Let's compare the atomic radii of several atoms to illustrate these principles:
-
Lithium (Li) vs. Beryllium (Be): Both are in the second period. Beryllium has a higher Zeff than Lithium, resulting in a smaller atomic radius for Beryllium.
-
Lithium (Li) vs. Sodium (Na): Both are in Group 1 (alkali metals). Sodium is in the third period, possessing a larger principal quantum number and a greater distance between its outermost electrons and the nucleus, leading to a significantly larger atomic radius for Sodium compared to Lithium.
-
Oxygen (O) vs. Sulfur (S): Oxygen and Sulfur are in Group 16 (chalcogens). Sulfur has a larger atomic radius because it lies below Oxygen in the periodic table, thus having an extra electron shell.
-
Chlorine (Cl) vs. Bromine (Br): Both are in Group 17 (halogens). Bromine has a larger atomic radius due to its position further down the group, resulting in more electron shells and a greater average distance between the outermost electrons and the nucleus.
Identifying the Atom with the Largest Atomic Radius
Pinpointing the single atom with the largest atomic radius requires careful consideration of the elements' position in the periodic table and the trends discussed above. While precise numerical values vary depending on the measurement method, the general trend is clear: elements at the bottom left of the periodic table possess the largest atomic radii.
Therefore, among all known elements, Francium (Fr) is generally considered to have the largest atomic radius. Its location in the bottom left corner of the periodic table, belonging to the alkali metal group, signifies it has the highest principal quantum number and the least effective nuclear charge among stable elements. This combination leads to the most significant distance between the nucleus and outermost electrons, resulting in the largest atomic radius.
It’s important to note that the radius of Francium is difficult to measure directly due to its high radioactivity and short half-life. However, extrapolations based on periodic trends strongly support its position as having the largest atomic radius amongst naturally occurring elements. Synthetic elements beyond Francium might theoretically have even larger radii, but their short lifespans and limited availability prevent conclusive measurements.
Conclusion: A Broader Perspective on Atomic Size
Determining the atom with the largest atomic radius is more than just a simple comparison of numbers. It necessitates a deep dive into the complexities of atomic structure and the interplay of various forces governing electron behavior. Understanding the principles of effective nuclear charge, electron shielding, and periodic trends provides a framework for predicting relative atomic sizes and appreciating the intricacies of the quantum world. While Francium emerges as the likely candidate for the largest atomic radius among stable elements, the principles discussed here offer valuable insights into the fascinating world of atomic structure and chemical properties. This knowledge forms the bedrock for numerous applications in chemistry, materials science, and other related fields.
Latest Posts
Latest Posts
-
What Unit Is Work Measured In
Mar 27, 2025
-
Is Boron A Gas Liquid Or Solid
Mar 27, 2025
-
What Is 20 Percent Of 12
Mar 27, 2025
-
What Percent Is 1 Of 7
Mar 27, 2025
-
When Gas Exerts Pressure On Its Container The Pressure Is
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Of The Following Which Atom Has The Largest Atomic Radius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
