Group 3 12 Elements Are Called
listenit
Mar 21, 2025 · 5 min read
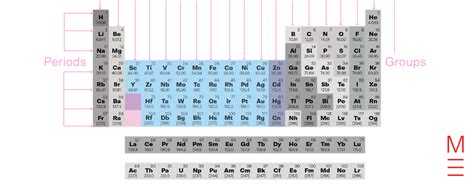
Table of Contents
Group 3: Unveiling the 12 Elements and Their Significance
Group 3, also known as Group IIIB in older nomenclature, holds a fascinating and somewhat controversial position in the periodic table. While definitively identifying the twelve elements encompassed within Group 3 requires careful consideration of varying definitions, understanding its complexities unlocks crucial insights into the nature of chemical behavior and the periodic system itself. This article delves deep into the composition, properties, and applications of these elements, addressing the nuances that define their classification.
Defining Group 3: A Matter of Perspective
The seemingly simple question, "What are the 12 elements in Group 3?" reveals a deeper debate within the scientific community. The traditional view, based on early periodic table arrangements, often included only scandium (Sc), yttrium (Y), and the lanthanides. However, a more contemporary perspective, aligned with the IUPAC (International Union of Pure and Applied Chemistry) recommendations, expands Group 3 to incorporate the entire d-block elements in the third column. This broader interpretation leads us to consider a twelve-element group.
This expanded view includes:
- Scandium (Sc): Atomic number 21
- Yttrium (Y): Atomic number 39
- Lutetium (Lu): Atomic number 71
- Lawrencium (Lr): Atomic number 103
And the elements of the 3rd transition series:
- Lanthanum (La): Atomic number 57
- Cerium (Ce): Atomic number 58
- Praseodymium (Pr): Atomic number 59
- Neodymium (Nd): Atomic number 60
- Promethium (Pm): Atomic number 61
- Samarium (Sm): Atomic number 62
- Europium (Eu): Atomic number 63
- Gadolinium (Gd): Atomic number 64
This expanded definition is based on the electronic configuration and the filling of the f orbitals which influences the chemical properties. Understanding this variance in classification is crucial for comprehending the discussions surrounding Group 3 elements.
Properties of Group 3 Elements: A Diverse Collection
The elements of Group 3, under the broader definition, exhibit a wide range of properties due to the variations in their electronic configurations and atomic sizes. While they share some similarities, particularly in their reactivity, the differences are significant.
Electronic Configuration and Chemical Behavior
The electronic configurations of these elements play a pivotal role in determining their chemical behavior. They all have at least one electron in the d orbital, which is critical for their metallic character and tendency to form cations (positively charged ions) by losing electrons. However, the specific number and arrangement of these d electrons, coupled with the presence or absence of f electrons in the lanthanides and actinides, lead to diverse chemical reactivity.
Metallic Properties
All elements within this expanded Group 3 are metals, characterized by their excellent electrical and thermal conductivity, malleability, and ductility. However, their specific mechanical properties vary widely, depending on the element’s position in the periodic table and the strength of metallic bonding. For example, scandium is relatively soft, while some lanthanides possess greater hardness.
Reactivity
The reactivity of Group 3 elements tends to increase down the group. Scandium and yttrium exhibit relatively high reactivity, readily reacting with oxygen and other nonmetals. The lanthanides also react with oxygen and nonmetals, though at variable rates. The actinides, especially lawrencium, are highly radioactive and their reactivity is influenced by their radioactivity.
Magnetic Properties
Many elements in Group 3 exhibit interesting magnetic properties. Some lanthanides, for instance, are paramagnetic, meaning they are weakly attracted to magnetic fields. Others exhibit ferromagnetism, strong attraction to magnetic fields, or even diamagnetism, a weak repulsion. This magnetic behavior arises from the unpaired electrons in their electronic configuration.
Applications of Group 3 Elements: A Spectrum of Uses
The applications of Group 3 elements are incredibly diverse and span many industries. Their unique properties dictate their suitability for various applications.
Scandium and Yttrium: Essential Components
Scandium and yttrium find crucial applications in high-tech industries. Scandium's addition to aluminum alloys significantly enhances their strength and heat resistance. It’s used in high-intensity discharge lamps (HID) producing exceptionally bright white light. Yttrium is a key component in high-temperature superconductors and is used in lasers (yttrium aluminum garnet, YAG lasers).
Lanthanides: Diverse Roles
The lanthanides boast an impressive range of applications, contributing to various technologies. Their use in catalysts is extensive, particularly in petroleum refining and the production of various chemicals. They are also integral to the manufacture of magnets, lighting components (fluorescent lamps), and certain alloys. Specific lanthanides play distinct roles. Neodymium magnets are exceptionally powerful, finding uses in wind turbines and hard disk drives. Europium's fluorescence is employed in color television screens.
Actinides: Radioactive Applications and Challenges
The actinides are all radioactive and their applications are largely confined to scientific research and nuclear applications. However, they also have a role in some specialized medical procedures. Their radioactivity presents considerable challenges in terms of handling and safety precautions. Lawrencium, being synthetic and extremely short-lived, is primarily used in nuclear physics research.
The Ongoing Research and Future Applications
Research into the Group 3 elements continues at a rapid pace. Scientists are actively exploring novel applications of these elements, focusing on areas such as:
- Advanced materials: The development of new alloys with enhanced properties for aerospace, automotive, and other industries.
- Catalysis: Discovering new catalysts for more efficient and sustainable chemical processes.
- Renewable energy: Utilizing lanthanides in more efficient solar cells and batteries.
- Biomedical applications: Exploring the potential of certain lanthanides in medical imaging and therapeutics.
- Nuclear energy: Further investigation into the potential of actinides in nuclear reactors and other energy applications.
Conclusion: A Complex and Crucial Group
Group 3, while seemingly simple in its designation, presents a complex and fascinating group of elements. Understanding the varied definitions, properties, and applications of these elements requires appreciating the nuances of the periodic table and the unique characteristics of each individual element. From their crucial roles in high-tech applications to their potential in shaping future technologies, the twelve elements of Group 3 hold significant importance in modern science and technology. Further research is poised to unlock even more of their potential and contribute to advancements across numerous scientific and industrial fields. Their contributions range from the creation of incredibly strong alloys to the advancement of medical technologies, making them integral to the modern world. The seemingly small column on the periodic table holds a wealth of potential, only now beginning to be fully explored.
Latest Posts
Latest Posts
-
4 To The Power Of Negative 1
Mar 28, 2025
-
What Is A Common Property Of Metals
Mar 28, 2025
-
What Does The Coefficient In A Chemical Equation Represent
Mar 28, 2025
-
1 4 Pound Is How Many Ounces
Mar 28, 2025
-
Oxidation State Of Nitrogen In Ammonia
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Group 3 12 Elements Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
