Oxidation State Of Nitrogen In Ammonia
listenit
Mar 28, 2025 · 6 min read
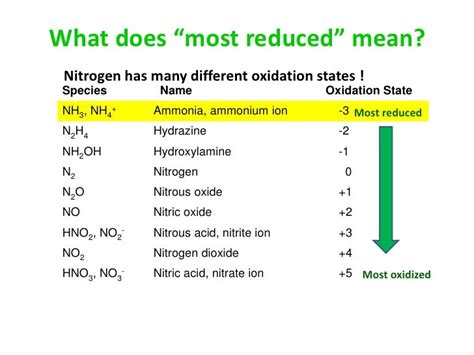
Table of Contents
The Oxidation State of Nitrogen in Ammonia: A Deep Dive
The oxidation state of nitrogen in ammonia (NH₃) is a fundamental concept in chemistry, crucial for understanding its reactivity and role in various chemical processes. While seemingly straightforward, a thorough examination reveals nuances and connections to broader chemical principles. This article provides a comprehensive exploration of the oxidation state of nitrogen in ammonia, delving into its calculation, implications, and significance in different contexts.
Understanding Oxidation States
Before focusing specifically on ammonia, let's establish a solid foundation in understanding oxidation states. The oxidation state, also known as the oxidation number, is a hypothetical charge assigned to an atom in a molecule or ion, assuming that all bonds are 100% ionic. This assignment is based on a set of rules, allowing us to track electron transfer during chemical reactions. It's a crucial tool for predicting reactivity, balancing redox reactions, and understanding chemical behavior.
Key Rules for Assigning Oxidation States:
- Free elements: The oxidation state of an atom in its elemental form is always zero (e.g., O₂ has an oxidation state of 0 for each oxygen atom).
- Monatomic ions: The oxidation state of a monatomic ion is equal to its charge (e.g., Na⁺ has an oxidation state of +1).
- Fluorine: Fluorine, being the most electronegative element, always has an oxidation state of -1 in its compounds.
- Oxygen: Oxygen typically has an oxidation state of -2 in its compounds, except in peroxides (like H₂O₂) where it's -1 and in compounds with fluorine (like OF₂) where it's positive.
- Hydrogen: Hydrogen usually has an oxidation state of +1 in its compounds, except in metal hydrides (like NaH) where it's -1.
- Sum of oxidation states: In a neutral molecule, the sum of the oxidation states of all atoms must equal zero. In a polyatomic ion, the sum of the oxidation states must equal the charge of the ion.
Calculating the Oxidation State of Nitrogen in Ammonia (NH₃)
Now, let's apply these rules to determine the oxidation state of nitrogen in ammonia (NH₃).
-
Hydrogen's oxidation state: In ammonia, hydrogen is bonded to a non-metal (nitrogen), so its oxidation state is +1. Since there are three hydrogen atoms, the total positive charge contribution from hydrogen is +3.
-
Overall charge: Ammonia (NH₃) is a neutral molecule, meaning its overall charge is zero.
-
Nitrogen's oxidation state: To satisfy the rule that the sum of oxidation states must equal zero, the oxidation state of nitrogen (N) must be -3. This ensures that (+3) + (-3) = 0.
Therefore, the oxidation state of nitrogen in ammonia is -3.
Implications of Nitrogen's -3 Oxidation State in Ammonia
The -3 oxidation state of nitrogen in ammonia has several crucial implications:
-
Ammonia's reducing properties: A negative oxidation state indicates that nitrogen in ammonia has a relatively high electron density. This makes ammonia a potent reducing agent, meaning it readily donates electrons to other species, undergoing oxidation itself in the process. This property is exploited in various industrial processes and chemical reactions. For instance, ammonia is used as a reducing agent in the production of some metals.
-
Ammonia's basicity: The lone pair of electrons on the nitrogen atom, resulting from its -3 oxidation state, makes ammonia a weak base. This lone pair can accept a proton (H⁺), forming the ammonium ion (NH₄⁺). This basicity is crucial in various applications, from cleaning solutions to biological systems.
-
Ammonia's stability: While ammonia can act as a reducing agent, it's relatively stable under normal conditions. The strong N-H bonds contribute to this stability, along with the low oxidation state of nitrogen. However, under specific conditions (e.g., high temperatures or presence of strong oxidizing agents), ammonia can undergo oxidation reactions.
-
Formation of other nitrogen compounds: The oxidation state of nitrogen can change through various chemical reactions involving ammonia. For example, the oxidation of ammonia can produce nitrogen oxides (NOₓ), nitric acid (HNO₃), and other nitrogen-containing compounds. The specific product formed depends on the oxidizing agent and reaction conditions.
Comparing Nitrogen's Oxidation State in Other Compounds
Comparing the oxidation state of nitrogen in ammonia (-3) to its oxidation states in other compounds highlights its versatility. Nitrogen exhibits a wide range of oxidation states, from -3 (in ammonia) to +5 (in nitric acid, HNO₃). This variable oxidation state is a key factor in nitrogen's diverse chemical behavior and its involvement in numerous biological and industrial processes.
Here's a comparison with some other common nitrogen compounds:
- Nitric oxide (NO): Nitrogen's oxidation state is +2.
- Nitrogen dioxide (NO₂): Nitrogen's oxidation state is +4.
- Nitric acid (HNO₃): Nitrogen's oxidation state is +5.
- Nitrous oxide (N₂O): Nitrogen's oxidation state is +1.
- Hydrazine (N₂H₄): Nitrogen's oxidation state is -2.
This variability in oxidation state contributes to nitrogen's importance in various chemical and biological processes. The -3 oxidation state in ammonia represents one extreme of this range, emphasizing its reducing capabilities and basic nature.
Ammonia's Role in Biological Systems
Ammonia, with its nitrogen in the -3 oxidation state, plays a vital role in biological systems. It's a crucial component of nitrogen metabolism, a process essential for all living organisms. Plants absorb ammonia from the soil, utilizing it to synthesize amino acids, proteins, and nucleic acids. Animals obtain nitrogen through the consumption of plants or other animals, breaking down proteins into ammonia and eventually excreting it in various forms (urea, uric acid). The nitrogen cycle, a complex biogeochemical process, involves the transformation of nitrogen between different oxidation states, highlighting the significance of ammonia as a starting point for many nitrogen-containing biomolecules.
Industrial Applications of Ammonia
The industrial applications of ammonia are vast and significant, leveraging both its reducing properties and its role as a nitrogen source. The Haber-Bosch process, a crucial industrial process, produces ammonia from nitrogen and hydrogen gases under high pressure and temperature, using a catalyst. This process is essential for the production of fertilizers, significantly impacting global food production. Ammonia is also used in the production of various chemicals, including nitric acid, nylon, and explosives.
Environmental Considerations
While ammonia is essential, its release into the environment can have negative consequences. Excessive ammonia in water bodies can lead to eutrophication, a process where nutrient enrichment causes excessive algal growth, depleting oxygen levels and harming aquatic life. Ammonia emissions from agricultural activities and industrial processes are significant environmental concerns, highlighting the need for sustainable practices to minimize its environmental impact.
Conclusion
The oxidation state of nitrogen in ammonia (-3) is far more than a simple numerical value; it's a key to understanding its fundamental chemical and biological properties. Its reducing power, basicity, and stability are all direct consequences of this oxidation state. Understanding the oxidation state of nitrogen in ammonia allows us to appreciate its significance in various industrial processes, biological cycles, and environmental considerations. Its seemingly simple chemical formula hides a wealth of complexity and impact, making it a truly fundamental component of chemistry and biology. Further exploration of the oxidation states of nitrogen in different compounds allows for a deeper understanding of its diverse roles in the chemical world.
Latest Posts
Latest Posts
-
Is H2s An Acid Or Base
Mar 31, 2025
-
What Is 83333 As A Fraction
Mar 31, 2025
-
Least Common Multiple 16 And 24
Mar 31, 2025
-
What Is The Name Of This Hydrocarbon
Mar 31, 2025
-
Columns Of Periodic Table Are Called
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Oxidation State Of Nitrogen In Ammonia . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
