Columns Of Periodic Table Are Called
listenit
Mar 31, 2025 · 7 min read
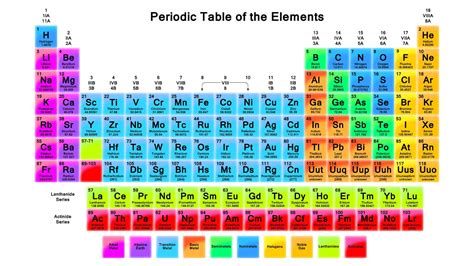
Table of Contents
Columns of the Periodic Table are Called Groups or Families: A Deep Dive
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its organization is crucial for comprehending chemical behavior and predicting reactions. A common question, especially for students beginning their chemistry journey, centers around the vertical columns of the periodic table. They are called groups or families. But what does this mean, and why is this arrangement so significant? This article will delve into the intricacies of groups within the periodic table, exploring their characteristics, trends, and importance in chemistry.
Understanding the Organization: Groups vs. Periods
The periodic table is a two-dimensional arrangement of elements. Elements are organized into:
-
Periods (Rows): These horizontal rows represent increasing atomic number and, consequently, the addition of another electron shell. Elements within the same period share the same number of electron shells.
-
Groups (Columns) or Families: These are the vertical columns, and this is where the elements share similar chemical properties. This similarity stems from the consistent number of valence electrons – the electrons in the outermost shell, which are primarily involved in chemical bonding.
The consistent number of valence electrons dictates how an element will react chemically. Elements in the same group will tend to form similar types of compounds and exhibit similar reactivity patterns. Understanding this fundamental principle is key to predicting chemical behavior.
The Significance of Valence Electrons
The reason groups possess similar properties is rooted in their valence electrons. These outermost electrons are responsible for the element's reactivity. They are the electrons that are most easily gained, lost, or shared during chemical reactions.
-
Group 1 (Alkali Metals): These elements have one valence electron, making them highly reactive. They readily lose this electron to form +1 ions, forming strong ionic bonds with nonmetals.
-
Group 2 (Alkaline Earth Metals): Possessing two valence electrons, these metals are less reactive than alkali metals but still readily lose electrons to form +2 ions.
-
Group 17 (Halogens): With seven valence electrons, halogens are highly reactive nonmetals. They tend to gain one electron to achieve a stable octet (eight electrons in their outermost shell), forming -1 ions and readily forming ionic bonds with metals.
-
Group 18 (Noble Gases): These elements have a full valence shell (eight electrons, except for helium with two), making them exceptionally unreactive. Their stable electron configuration explains their inertness.
This pattern continues throughout the periodic table, demonstrating the crucial role valence electrons play in determining an element's chemical behavior and its position within a group.
Predicting Properties Based on Group Membership
The periodic table's arrangement allows us to predict the properties of elements based solely on their group. For example:
-
Reactivity: Elements in the same group generally exhibit similar reactivity. Knowing that lithium (Li) is highly reactive, we can reasonably predict that sodium (Na), potassium (K), and other alkali metals in Group 1 will also be highly reactive.
-
Melting and Boiling Points: Trends in melting and boiling points often exist within a group. For example, melting and boiling points generally increase as you move down Group 18 (noble gases) due to increasing interatomic forces.
-
Ionization Energy: This refers to the energy required to remove an electron from an atom. Elements within the same group generally exhibit similar trends in ionization energy. For example, ionization energy typically decreases as you move down a group.
-
Electronegativity: This describes an atom's ability to attract electrons in a chemical bond. Trends in electronegativity are also evident within groups.
By understanding these trends and relationships within groups, chemists can predict the behavior of elements, design experiments, and understand the formation and properties of compounds.
The Importance of Group Numbering
The numbering of groups has undergone revisions over time, leading to some confusion. Two main systems are in common use:
-
Traditional Group Numbers (IA, IIA, etc.): This older system used Roman numerals and letters (IA, IIA, IIIA, etc.) to denote groups. While still used in some contexts, it is gradually being replaced.
-
Modern Group Numbers (1, 2, 13-18): This system uses Arabic numerals (1, 2, 13-18), reflecting the current understanding of the underlying electron configurations. This is the system preferred by IUPAC (International Union of Pure and Applied Chemistry).
Regardless of the numbering system, the core concept remains the same: elements in the same group share similar properties due to similar valence electron configurations.
Exploring Specific Groups in Detail
Let's examine some key groups in more detail to illustrate the significance of group membership:
Group 1: Alkali Metals
These highly reactive metals are characterized by their single valence electron. They are soft, silvery-white metals with low melting points. Their reactivity stems from their tendency to readily lose their valence electron, forming +1 ions. Their reactions with water are particularly vigorous, producing hydrogen gas. Examples include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
Group 2: Alkaline Earth Metals
Slightly less reactive than alkali metals, alkaline earth metals possess two valence electrons. They are also silvery-white metals but are harder and have higher melting points than alkali metals. They form +2 ions. Examples include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
Group 17: Halogens
These highly reactive nonmetals are characterized by their seven valence electrons. They are diatomic (existing as two-atom molecules) and readily gain one electron to form -1 ions. Their reactivity decreases down the group. Examples include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
Group 18: Noble Gases
These elements possess a full valence shell, making them exceptionally unreactive (inert). They are colorless, odorless gases with low boiling points. Their stability is due to their complete octet (eight valence electrons), satisfying the octet rule. Examples include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
Transition Metals (Groups 3-12):
These elements occupy the central block of the periodic table. They are characterized by their partially filled d orbitals and variable oxidation states (meaning they can lose different numbers of electrons to form ions with different charges). This variable oxidation state is responsible for the diverse chemical behavior of these elements. They are often involved in forming coordination compounds and exhibit catalytic properties.
Inner Transition Metals (Lanthanides and Actinides):
These elements are placed separately at the bottom of the periodic table. The lanthanides fill their 4f orbitals, while the actinides fill their 5f orbitals. They exhibit similar chemical properties within their respective series and often occur together in nature.
Beyond the Basic Trends: Exceptions and Nuances
While the trends within groups are generally reliable, exceptions and nuances exist. For example, the first element in a group often displays different properties compared to its heavier congeners (elements below it in the same group). This is due to its smaller size and higher ionization energy. Additionally, relativistic effects can become significant for heavier elements, influencing their properties. Understanding these exceptions is crucial for a complete understanding of the periodic table.
The Periodic Table: A Powerful Predictive Tool
The organization of the periodic table into groups and periods provides chemists with a powerful predictive tool. Understanding the underlying principles governing the arrangement – particularly the significance of valence electrons – allows for the prediction of properties, reactivity, and chemical behavior. This knowledge underpins much of our understanding of the world around us and is critical to advances in various fields, including material science, medicine, and environmental science.
Conclusion
The columns of the periodic table, known as groups or families, are not just a convenient organizational system. They represent a fundamental understanding of the underlying structure and behavior of elements. The shared properties within a group stem from the consistent number of valence electrons, dictating their reactivity and chemical interactions. By understanding the relationships and trends within groups, chemists can predict chemical behavior, design new materials, and advance our knowledge of the chemical world. The periodic table, therefore, serves as a cornerstone of modern chemistry, facilitating both fundamental research and technological advancement.
Latest Posts
Latest Posts
-
Why Are Hydrogen Bonds Important For Life
Apr 01, 2025
-
How Many Oz In Quarter Pound
Apr 01, 2025
-
How Many Cc In 10 Ml
Apr 01, 2025
-
Number Of Valence Electrons For Silicon
Apr 01, 2025
-
What Is The Area Of This Circle In Square Centimeters
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Columns Of Periodic Table Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
