Number Of Valence Electrons For Silicon
listenit
Apr 01, 2025 · 5 min read
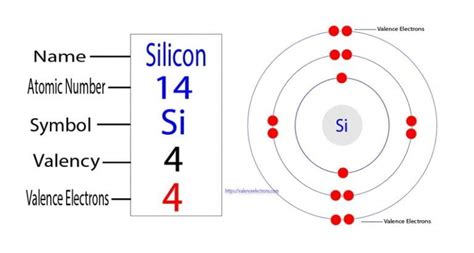
Table of Contents
Delving Deep into Silicon: Unveiling the Significance of its Four Valence Electrons
Silicon, the second most abundant element in the Earth's crust, plays a pivotal role in modern technology. Its unique properties, largely stemming from its electronic configuration, make it indispensable in semiconductors, solar cells, and countless other applications. Understanding the number of valence electrons in silicon is crucial to grasping its chemical behavior and technological significance. This article will explore silicon's valence electrons in detail, examining their impact on its bonding, conductivity, and ultimately, its multifaceted applications.
Understanding Valence Electrons: The Key to Chemical Behavior
Before diving into silicon specifically, let's establish a fundamental understanding of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons directly dictates an element's position in the periodic table and its group properties. For instance, elements in Group 1 (alkali metals) have one valence electron, while those in Group 18 (noble gases) have a full valence shell, making them largely unreactive.
Silicon's Position in the Periodic Table and its Electronic Configuration
Silicon (Si) resides in Group 14 (also known as Group IVA) of the periodic table. Its atomic number is 14, indicating it possesses 14 protons and, in its neutral state, 14 electrons. The electronic configuration of silicon is 1s²2s²2p⁶3s²3p². This configuration reveals the crucial information we need: silicon has four valence electrons. These four electrons are located in the outermost shell (the third shell, specifically in the 3s and 3p orbitals).
The Significance of Four Valence Electrons: Bonding and Semiconducting Properties
The presence of four valence electrons is what distinguishes silicon and gives it its remarkable properties. This number allows silicon to form stable covalent bonds with a variety of elements, significantly influencing its applications in diverse fields.
Covalent Bonding in Silicon: A Strong Foundation
Silicon's four valence electrons participate in covalent bonding, meaning it shares electrons with other atoms to achieve a stable octet (eight electrons) in its outermost shell. This sharing results in strong, directional bonds. In pure silicon, each silicon atom bonds covalently with four neighboring silicon atoms, forming a three-dimensional network of interconnected atoms. This tetrahedral arrangement is the foundation of silicon's crystalline structure and its mechanical strength.
Silicon's Semiconducting Nature: Bridging the Gap between Conductors and Insulators
The unique bonding arrangement of silicon leads to its semiconducting properties, a crucial characteristic for its role in modern electronics. While a pure silicon crystal has a relatively low conductivity at room temperature, its conductivity can be significantly altered by doping – the introduction of impurity atoms.
Doping: Enhancing Silicon's Conductivity
-
N-type doping: Introducing elements with five valence electrons (like phosphorus or arsenic) into the silicon lattice creates extra electrons. These extra electrons become mobile charge carriers, increasing the silicon's conductivity. These extra electrons are often referred to as majority carriers.
-
P-type doping: Introducing elements with three valence electrons (like boron or aluminum) creates "holes" – the absence of an electron in the silicon lattice. These holes act as positive charge carriers, also enhancing conductivity. The holes are considered the majority carriers in p-type semiconductors.
The combination of n-type and p-type silicon forms the basis of p-n junctions, which are fundamental components in transistors, diodes, and integrated circuits. The ability to precisely control silicon's conductivity through doping is the cornerstone of modern electronics.
Applications of Silicon: Leveraging its Unique Properties
Silicon's unique combination of properties, driven by its four valence electrons, has led to its widespread use in numerous technological applications.
Semiconductors: The Heart of Modern Electronics
Silicon's semiconducting properties are paramount to the semiconductor industry. Transistors, integrated circuits (ICs), microprocessors, and memory chips are all built upon silicon's ability to control and manipulate electrical current flow. The miniaturization of electronics has largely been made possible by silicon's ability to be easily doped and fabricated into intricate structures.
Solar Cells: Harnessing the Sun's Energy
Silicon's interaction with light is another key application. Crystalline silicon solar cells convert sunlight into electricity. The photovoltaic effect, where photons excite electrons in the silicon, leading to the flow of current, is a direct result of silicon's electronic structure. The four valence electrons enable the efficient absorption of sunlight and the generation of electricity.
Other Applications of Silicon
Beyond semiconductors and solar cells, silicon finds use in a wide array of applications:
-
Silicones: Silicon-based polymers, silicones, exhibit remarkable thermal stability and water repellency, making them useful in lubricants, sealants, and cosmetics.
-
Ceramics: Silicon carbide (SiC) is a very hard ceramic material used in high-temperature applications and cutting tools. Its strength is directly related to the strong covalent bonds formed by silicon's four valence electrons.
-
Glass: Silicon dioxide (SiO2), or silica, is the primary component of glass, a material crucial in various applications, from windows to optical fibers.
-
Metallurgy: Silicon is used as an alloying agent in various metals, improving their properties like strength and castability.
The Future of Silicon: Continued Innovation and Research
Despite the vast progress made in silicon technology, research continues to explore new possibilities and push the boundaries of silicon's capabilities. Scientists are constantly striving to improve silicon's efficiency in solar cells, enhance its performance in transistors, and explore novel applications. The ongoing efforts to refine silicon-based materials and devices highlight the ongoing significance of understanding its fundamental properties, rooted in its four valence electrons.
Conclusion: A Deep Dive into the Significance of Four Valence Electrons
The number of valence electrons an atom possesses fundamentally dictates its chemical and physical properties. Silicon, with its four valence electrons, exhibits a unique combination of properties, making it an indispensable element in modern technology. Its ability to form strong covalent bonds, its semiconducting nature, and its versatility in various applications are all directly linked to this crucial aspect of its electronic structure. Understanding the significance of silicon's four valence electrons provides a crucial framework for appreciating its vast applications and future potential in countless technological advancements. The continued research and development in silicon-based technologies showcase the ongoing relevance of this element and its profound impact on our daily lives. The exploration of silicon's properties continues to be a fascinating area of study, promising even more exciting developments in the future.
Latest Posts
Latest Posts
-
Which Two Subatomic Particles Are Located In The Nucleus
Apr 02, 2025
-
Irrational Numbers Between 1 And 6
Apr 02, 2025
-
In A Hypertonic Solution A Cell Will
Apr 02, 2025
-
How Many Kilograms Are In 5000 Grams
Apr 02, 2025
-
Solve This Inequality 3b 7 32
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons For Silicon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
