What Does The Coefficient In A Chemical Equation Represent
listenit
Mar 28, 2025 · 5 min read
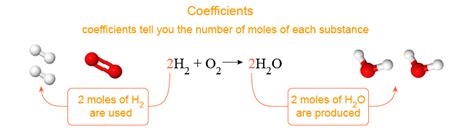
Table of Contents
What Does the Coefficient in a Chemical Equation Represent?
Understanding chemical equations is fundamental to grasping the principles of chemistry. These equations, at their core, represent chemical reactions, showing us the reactants transforming into products. A crucial element within these equations is the coefficient, often overlooked but essential for interpreting the reaction's quantitative aspects. This article delves deep into the meaning and significance of coefficients in chemical equations, exploring their implications for stoichiometry, balancing equations, and interpreting reaction yields.
The Foundation: Understanding Chemical Equations
Before we delve into the specifics of coefficients, let's establish a foundational understanding of chemical equations. A chemical equation uses chemical formulas to describe a chemical reaction. It shows the reactants (the starting materials) on the left side of an arrow and the products (the substances formed) on the right side.
For example, the combustion of methane can be represented as:
CH₄ + 2O₂ → CO₂ + 2H₂O
In this equation:
- CH₄ (Methane): Represents one molecule of methane, a reactant.
- 2O₂ (Oxygen): Represents two molecules of oxygen, also a reactant.
- → (Arrow): Indicates the transformation from reactants to products.
- CO₂ (Carbon Dioxide): Represents one molecule of carbon dioxide, a product.
- 2H₂O (Water): Represents two molecules of water, another product.
This seemingly simple equation holds a wealth of information about the reaction, and the coefficients are key to unlocking it.
The Significance of Coefficients: The Mole Ratio
The coefficients in a balanced chemical equation represent the relative number of moles of each reactant and product involved in the reaction. They are crucial for stoichiometry, the quantitative relationship between reactants and products in a chemical reaction.
Let's revisit the methane combustion example:
CH₄ + 2O₂ → CO₂ + 2H₂O
The coefficients tell us that:
- One mole of methane reacts with two moles of oxygen.
- This reaction produces one mole of carbon dioxide and two moles of water.
It's crucial to remember that coefficients represent moles, not molecules or grams. While we can conceptually visualize molecules, coefficients are about the macroscopic quantities involved in chemical reactions, typically measured in moles.
Beyond Moles: Interpreting Coefficients in Different Contexts
The mole ratio provided by the coefficients is the foundation for various stoichiometric calculations. However, the interpretation can be extended to other quantitative representations:
- Mole Ratios: This is the most direct interpretation, as previously discussed. It allows for direct comparisons and calculations between the quantities of reactants and products.
- Mass Ratios: By using the molar mass of each substance, you can convert the mole ratios to mass ratios, enabling calculations involving grams or kilograms.
- Volume Ratios (For Gases): If the reaction involves gases at the same temperature and pressure, the coefficients also represent the volume ratios of the gases. This is based on Avogadro's Law, stating that equal volumes of gases at the same temperature and pressure contain the same number of molecules.
- Number of Particles: While not often explicitly used in calculations, the coefficients can represent the relative number of molecules, atoms, or ions involved in the reaction. However, this is less practical in large-scale chemical processes.
Balancing Chemical Equations: The Role of Coefficients
Balancing a chemical equation ensures that the law of conservation of mass is obeyed. This law states that matter cannot be created or destroyed in a chemical reaction; the total mass of the reactants must equal the total mass of the products. Achieving this balance requires adjusting the coefficients to ensure that the number of atoms of each element is the same on both sides of the equation.
Consider the incomplete combustion of methane:
CH₄ + O₂ → CO + H₂O
This equation is unbalanced. To balance it, we adjust the coefficients:
2CH₄ + 3O₂ → 2CO + 4H₂O
Now, we have:
- 4 carbon atoms on both sides
- 8 hydrogen atoms on both sides
- 6 oxygen atoms on both sides
The equation is now balanced, accurately reflecting the conservation of mass during the reaction.
Coefficients and Limiting Reactants
In many reactions, one reactant is completely consumed before others. This reactant is called the limiting reactant, and it dictates the maximum amount of product that can be formed. Coefficients are essential in identifying the limiting reactant and calculating the theoretical yield.
Let's imagine reacting 2 moles of CH₄ with 3 moles of O₂ in the balanced combustion equation:
CH₄ + 2O₂ → CO₂ + 2H₂O
Based on the coefficients:
- 1 mole of CH₄ requires 2 moles of O₂.
- 2 moles of CH₄ would require 4 moles of O₂.
Since only 3 moles of O₂ are available, O₂ is the limiting reactant. The amount of CO₂ and H₂O produced will be limited by the available oxygen.
Coefficients and Percent Yield
The theoretical yield is the maximum amount of product that could be produced based on stoichiometry. However, in real-world reactions, the actual yield is often less than the theoretical yield due to various factors, such as incomplete reactions, side reactions, and losses during the process. The percent yield indicates the efficiency of a reaction:
Percent Yield = (Actual Yield / Theoretical Yield) x 100%
Coefficients are essential for calculating the theoretical yield, which is the basis for determining the percent yield. A high percent yield indicates a more efficient reaction, while a low percent yield suggests potential issues in the reaction process.
Advanced Applications of Coefficients
Beyond basic stoichiometry, coefficients play a role in more advanced chemical concepts:
- Equilibrium Constant (K): The coefficients in a balanced chemical equation are exponents in the expression for the equilibrium constant, influencing the magnitude of K and the position of equilibrium.
- Rate Laws: Coefficients do not directly determine reaction rates, but they are used to derive stoichiometric relationships between rates of consumption of reactants and formation of products.
- Thermochemistry: Coefficients are used in calculations involving enthalpy changes (ΔH), entropy changes (ΔS), and Gibbs free energy changes (ΔG) for reactions.
Conclusion: The Underrated Power of Coefficients
Coefficients in a chemical equation might seem like a minor detail, but their significance is immense. They are the bridge between the symbolic representation of a chemical reaction and its quantitative reality. Understanding their meaning—representing the relative number of moles of reactants and products—is fundamental to mastering stoichiometry, predicting reaction yields, identifying limiting reactants, and delving deeper into more advanced chemical concepts. By appreciating the power of coefficients, you gain a more comprehensive understanding of the language of chemistry and its quantitative underpinnings. They are a cornerstone for accurately representing and interpreting the behavior of chemical systems.
Latest Posts
Latest Posts
-
The Sum Of 3 Consecutive Integers
Mar 31, 2025
-
Is Benzene A Pure Substance Or Mixture
Mar 31, 2025
-
What Is 1 4 3 8 Reduced To The Lowest Terms
Mar 31, 2025
-
Least Common Multiple Of 12 And 40
Mar 31, 2025
-
How Do You Find Change In Velocity
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Does The Coefficient In A Chemical Equation Represent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
